The skin microbiome of patients with atopic dermatitis (AD) exhibits reduced bacterial diversity. The pathogen Staphylococcus aureus (S. aureus) often predominates. This gram-positive bacterium intensifies inflammatory processes and is associated with acute AD episodes. A study published in 2024 in JCI Insight by Clowry et al. provides insights into the systemic and cutaneous immune signatures associated with S. aureus-infectedpediatric AD.
For the study by Clowry et al. 93 AD patients aged 0-16 years were recruited [6]. Of these, 12 had a confirmed S. aureus skin infectionbased on clinical criteria (“ADS.aureus”) [7]. The clinical criteria included oozing, pustule formation, abscesses and/or crust formation as well as positive bacterial smear results. The remaining 46 AD patients did not meet the clinical criteria for the diagnosis of S. aureus skin infection(AD control group). The skin-healthy control group consisted of 35 participants who had neither an active S. aureus skin infectionnor a history of atopy.
S. aureus skin infectionwas associated with higher AD severity
Systemic immunological profiles were created for all 93 patients and, in addition, local skin immune profiles were created for a subgroup (n=69) [6]. This subgroup included 9 “ADS.aureus” patients, 32 patients from the AD control group and 28 skin-healthy controls. “AD.aureus” patients had a higher AD severity compared to the AD control group (mean EASI scores of 29.4-32 compared to 14.8-15). In addition, a greater number of patients in the “ADS.aureus” group were affected by high levels of S. aureus colonizationin both lesional (67% and 37%, respectively) and non-lesional (30% and 5%, respectively) skin. All patients in the “ADS.aureus” group had moderate or high levels of S. aureus on the lesional skin, 60% had colonization of the non-lesional skin and 66% had nasal colonization. In contrast, the AD control group showed no clinical signs of S. aureus infection. In 51% of these patients, S. aureus was identified on the lesional AD skin, in 30% on the non-lesional AD skin and 48% had nasal colonization. “AD.aureus” patients were less likely to be taking immunomodulatory systemic drugs (8-11% compared to 41-46% in the AD control group).
| Microbiome research adds an important level to the knowledge of pathogenetic processes in AD. It is now known that the cutaneous microbiota can influence and modulate immune responses in the skin, with S. aureus appearing to play a key role [1]. While S. aureus is only detectable on healthy skin in <5% of all swabs, it is found in children with AD in >90% of cases [2]. The already weakened barrier function of the skin in AD can be further impaired by S. aureus , as the bacterium has proteases that further reduce the integrity of the barrier [3]. This is because the protease activity enables penetration into the epidermis, which results in stimulation of Th2 cytokines, so that S. aureus can be found not only in the epidermis but also in the dermis of AD lesions [4]. Furthermore, S. aureus expresses an α-toxin – a pore-forming protein that damages the cell membrane of keratinocytes [5]. |
Immunological profiles of “ADS.aureus” vs. AD control group?
The initial analysis focused on the totality of “AD.aureus” antigen-specific memory T cell responses [6]. Skin-selective homing of memory and effector T cells is an important immunologic process in the pathophysiology of AD. Cutaneous lymphocyte-associated antigen (CLA) is a skin homing receptor that defines a subset of circulating memory T cells To better understand the skin-directed “AD.aureus” antigen-specific memory T cell responses, the staining panel was expanded to include CLA [8].
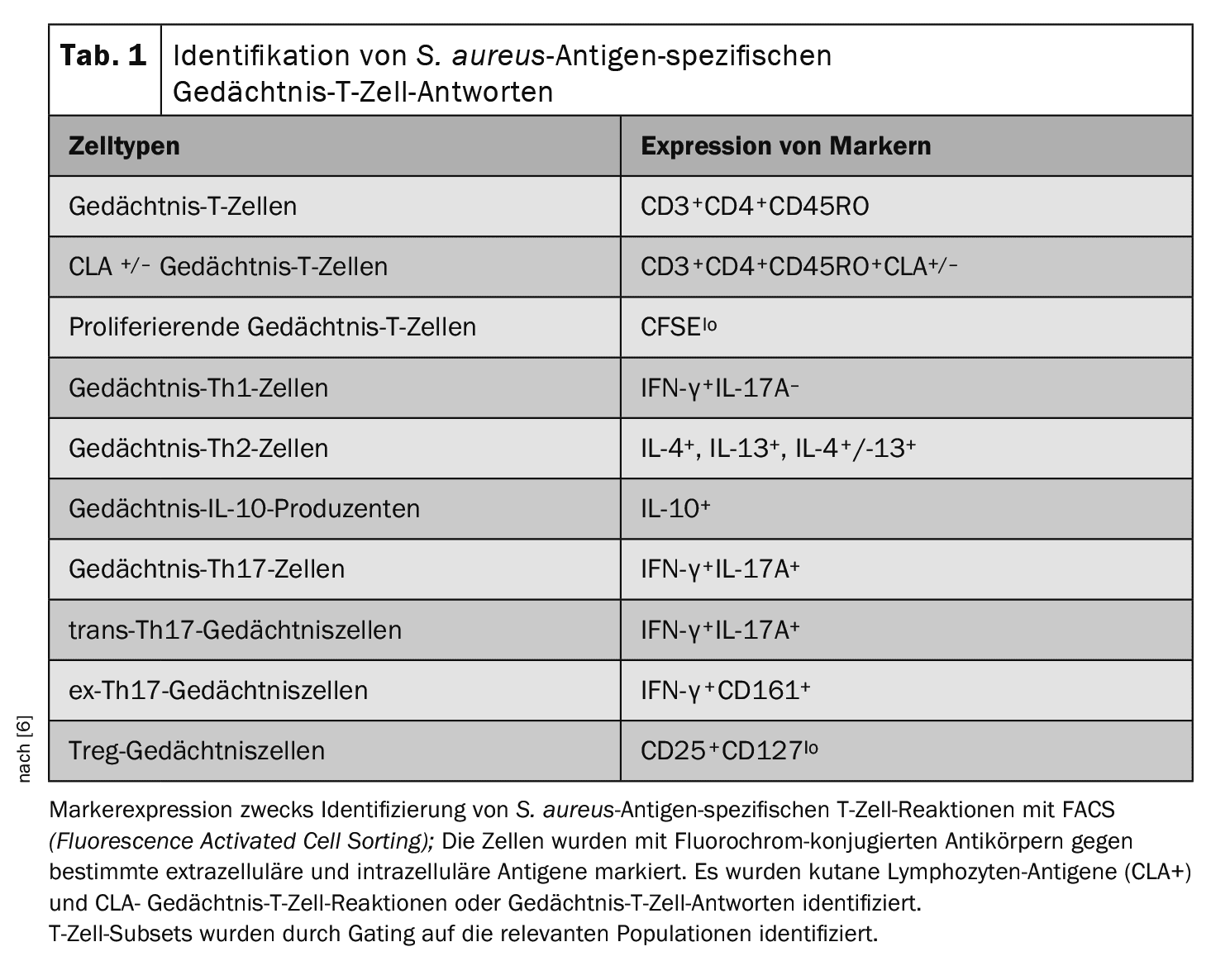
Characterization of circulating T-cells and S. aureus antigen-specificT-cell responses associated with S. aureus-infectedrelapses: To determine whether different circulating T-cell phenotypes and S. aureus antigen-specificT cell responses can be identified between “ADS.aureus”, the AD control group and the skin-healthy controls, peripheral blood mononuclear cells (PBMCs) were collected from all 93 recruited patients [6]. Circulating leukocyte populations were analyzed to determine the proportion of specific T-cell subsets by flow cytometric staining. To assess S. aureus antigen-specificsystemic responses, total PMBCs were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured in vitro in the presence of heat-inactivated S. aureus (strain AD08). Cells stimulated with the medium alone or with staphylococcal enterotoxin A (SEA) represented negative and positive controls, respectively. On day 8, cells were collected and stained with a series of fluorochrome-conjugated antibodies against surface and intracellular markers (Table 1). Fluorescence-activated cell sorting (FACS) was used to identify the specified T cell subsets.
The proportions of circulating systemic T-cell subsets and S. aureus antigen-specificmemory T-cell subsets identified in the three groups were entered into a Bayesian multinomial model.
- Analysis results: The feature with the highest probability of distinguishing “AD.aureus” from AD controls was IL-10-producing memory T cells with a mean probability of more than 80%, followed by circulating Th1 cells with a mean probability of 75% and circulating Vδ2+ cells with a mean probability of 63%. Further distinguishing features were circulating CD8+, CD4+, Vδ1+ and Th2 cells as well as ex-Th17 memory cells (a functionally different subset of Th17 cells that no longer produce IL-17 but IFN-γ), whose median probability of differentiation was between 50% and 60%.
- To determine the directionality of the traits with the highest probability of association with “AD.aureus” (>50%), ridge plots were used to compare the expression of the eight most important variables in “AD.aureus” compared to AD controls. Memory IL-10+ and circulating Th1 cells were suppressed in “ADS.aureus” compared to the AD control group, while circulating Vδ2+ cells were increased in “ADS.aureus” compared to the AD controls. In addition, circulating CD8+, CD4+ and ex-Th17 memory cells were also suppressed in “ADS.aureus”, while circulating Th2 and Vδ1+ cells were increased.
Characterization of antigen-specific T cell responses of S. aureus in the skin and of local inflammatory markers associated with S. aureus-infectedflares: By adding CLA to the staining panel, S. aureus antigen-specificmemory T cells could be identified in the skin [8]. CLA is a skin-specific adhesion molecule and represents a post-translational modification of P-selectin ligand-1 (PSGL-1) [9]. The CLA epitope binds specifically to E-selectin on the endothelium of the postcapillary venules and enables the selective migration of T lymphocytes from the peripheral circulation into the dermis [10].
| Summary |
| In the pediatric AD cohort studied by Clowry et al. pediatric AD cohort, S. aureus skin infectionwas most strongly associated with an increase in the cutaneous chemokines IP10 and TARC, which preferentially direct Th1 and Th2 cells to the skin [6]. Systemic CD4+ and CD8+ T cells, with the exception of Th2 cells, were suppressed in S. aureus skin infection, especially circulating Th1 cells, IL-10+ memory T cells and skin-resident Th17 memory cells. In addition, systemic γδ T cell expansion was observed in patients with S. aureus skin infection. |
| The increase in both circulating Th2 cells in the systems-only analysis and the S. aureus antigen-specific IL-4+IL-13+ (Th2) memory response in the combined analysis reflects the Th2 signature that characterizes AD [12] and is enhanced by S. aureus [13,14]. |
| The CLA-IL-4+IL-13+ S. aureus antigen-specificTh2 memory response had a higher probability of association with “ADS.aureus”. This could be due to homing by the CLA+IL-4+IL-13+ population. Interestingly, only dual IL-4- and IL-13-producing T lymphocytes were consistently elevated in “ADS.aureus” compared to AD controls. |
| The results of this work suggest that augmentation of protective subsets of T cells is a potential therapeutic strategy to reduce S. aureus dominancein AD. |
Cytokine profiles of non-lesional stratum corneum tape strips were also generated to identify local inflammatory responses in the skin [11]. Skin cytokine profiles associated with skin-specific S. aureus antigen responseswere available for a subset of 69 patients and were analyzed along with the associated systemic circulating T-cell profiles for these patients using the Bayesian multinomial model.
- Results of analysis: As before, the results identified both systemic and local cutaneous T cell-mediated immune responses that discriminated between “AD.aureus”, AD controls and skin-healthy controls with the highest discriminatory power. The addition of systemic skin responses and markers of cutaneous inflammatory response increased the number of features that discriminated between “AD.aureus”, AD controls and skin-healthy controls with greater than 50% confidence from 8 to 30, suggesting that the addition of these additional features provides greater insight into the local immune profiles associated with “AD.aureus”.
Literature:
- Lee HJ, Kim M: Skin Barrier Function and the Microbiome. Int J Mol Sci 2022 Oct 28; 23(21): 13071. doi: 10.3390/ijms232113071.
- Schöfer H, et al.: S2k + IDA guideline: Diagnosis and treatment of Staphylococcus aureus infections of the skin and mucous membranes. 2011. https://dgpi.de/wp-content/uploads/2013/04/013-038l_S2k_
Staphyococcus_aureus_2011-09.pdf, (last accessed 02.08.2024). - Fölster-Holst R: Die Rolle des Hautmikrobioms bei atopischer Dermatitis – Zusammenhänge und Konsequenzen. JDDG 2022; 20(5): 571-578.
- Nakatsuji T, et al: Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J Invest Dermatol 2016; 136: 2192-2200.
- Brauweiler AM, Goleva E, Leung DYM: Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol 2014; 134: 2114-2121.
- 6 Clowry J, et al: Distinct T cell signatures are associated with Staphylococcus aureus skin infection in pediatric atopic dermatitis. JCI Insight 2024 Apr 11; 9(9): e178789.
- Alexander H, et al: The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol 2020; 182(6): 1331-1342.
- de Jesús-Gil C, et al: The translational relevance of human circulating memory cutaneous lymphocyte-associated antigen positive T cells in inflammatory skin disorders. Front Immunol. 2021; 12: 652613. doi: 10.3389/fimmu.2021.652613.
- Fuhlbrigge RC, et al: Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 1997; 389(6654): 978-981.
- Czarnowicki T, et al. Circulating CLA+ T cells in atopic dermatitis and their possible role as peripheral biomarkers. Allergy 2017; 72(3): 366-372.
- Andersson AM, et al: Assessment of biomarkers in pediatric atopic dermatitis by tape strips and skin biopsies. Allergy 2022;77(5):1499-1509.
- Czarnowicki T, et al: Atopic dermatitis endotypes and implications for targeted therapeutics. JACI 2019; 143(1): 1-11.
- Karauzum H, Datta SK: Adaptive immunity against staphylococcus aureus. Curr Top Microbiol Immunol 2017; 409: 419-439.
- Geoghegan JA, et al: Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol 2018; 26(6): 484-497.
DERMATOLOGY PRACTICE 2024; 34(4): 26-27