Thyroid carcinomas can be diagnosed well today thanks to modern imaging techniques and often have a good prognosis. However, postoperative risk stratification should take place at a center with a dedicated thyroid board.
Modern imaging techniques are increasingly used to diagnose thyroid nodules. Most of these nodules are benign. A precise workup and analysis are therefore indispensable to avoid unnecessary surgical interventions. For suspicious thyroid nodules, fine needle biopsy and cytologic workup is the gold standard for diagnosis. If a carcinoma of the thyroid gland is confirmed cytologically or histologically, it is recommended to discuss the findings in an interdisciplinary thyroid board as the therapeutic options become more complex. Consensus can then be reached to determine the optimal therapy and follow-up for the patient. This article provides an overview of the diagnosis and treatment of thyroid carcinoma. The emphasis here is placed on therapy. The current international guidelines serve as a basis.
Epidemiology
Thyroid carcinoma is a rare tumor with low malignancy potential. It is the most common endocrine tumor. In Switzerland, about 770 new cases are recorded per year, which corresponds to 1.9% of all carcinomas. Women are more commonly affected than men in a ratio of 2.5:1 [3].
The mortality of thyroid carcinoma is about 0.4% of all malignant tumors. Papillary and follicular thyroid carcinoma have 5-year overall survival rates of nearly 100% and 97%, respectively, with optimal therapy. However, some histologic subtypes show a much worse prognosis with 10-40% mortality over 10 years. For medullary thyroid carcinomas, the 5-year overall survival rate is 90%. In the case of metastases, the prognosis is worse. In contrast to differentiated thyroid carcinoma, the prognosis for anaplastic or undifferentiated thyroid carcinoma is extremely poor with a 5-year overall survival rate of 7% [1].
Papillary thyroid carcinoma is the most common carcinoma of the thyroid gland with an incidence of 60-80%, followed by follicular, medullary, and anaplastic/undifferentiated thyroid carcinoma. The latter is a rarity, accounting for less than 2% of all thyroid carcinomas.
The incidence of papillary thyroid carcinoma is increasing for small papillary carcinomas <2 cm, but incidence and mortality is constant for larger ones. This can be explained by a more frequent diagnosis of clinically non-manifest thyroid nodules. The prevalence of clinically nonmanifest thyroid nodules increases with increasing age of the population. In screening studies, the prevalence of thyroid nodules exceeds 50% in patients older than 60 years [6].
Diagnosis
Clinical examination: If a thyroid nodule is suspected, a brief clinical examination of the soft tissues of the neck is recommended. Thyroid nodules larger than 1.5 cm can usually be palpated well. Clinical diagnosis also includes lymph node status. Undisplaced lymph nodes, ipsilateral and localized in the lower neck regions, are suspect. New-onset hoarseness may also be a sign of malignancy.
Laboratory chemical diagnostics: In the clarification of thyroid nodules with regard to malignancy, the thyroid hormone status plays a subordinate role, because the values say nothing about the benignity or malignancy of a nodule. Thyroglobulin is an excellent tumor progression parameter postoperatively in thyroid carcinoma. However, non-specific thyroglobulin is unsuitable for diagnosis. The situation is quite different with serum calcitonin as a tumor parameter for medullary thyroid carcinoma. In some centers, serum calcitonin is therefore routinely determined in the workup of thyroid nodules.

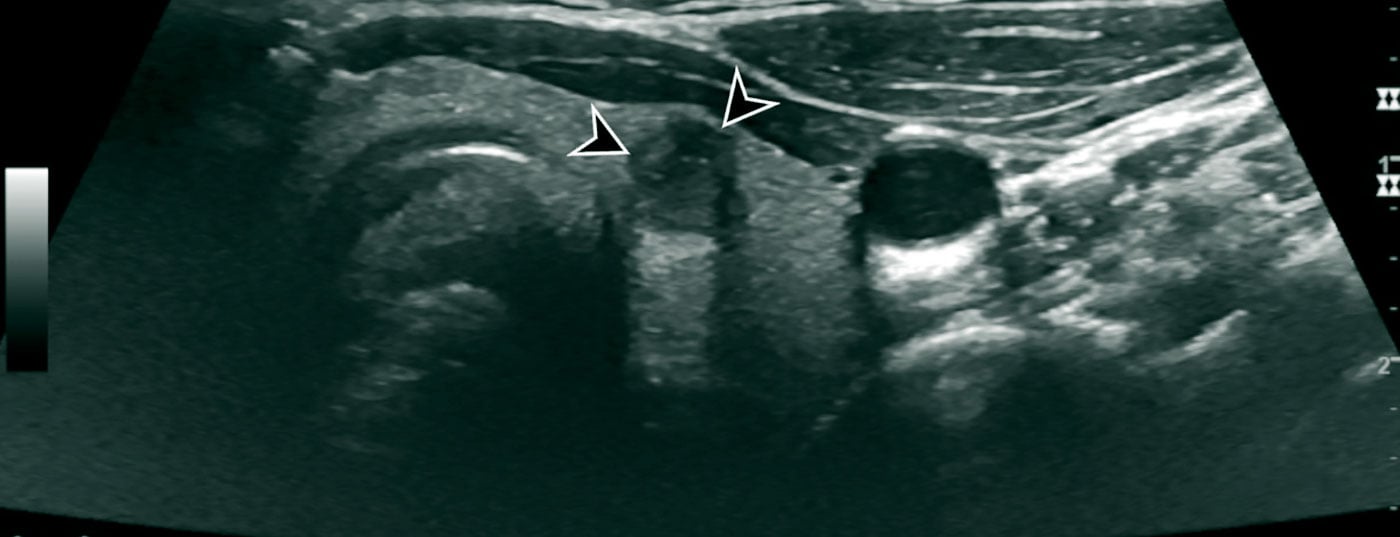
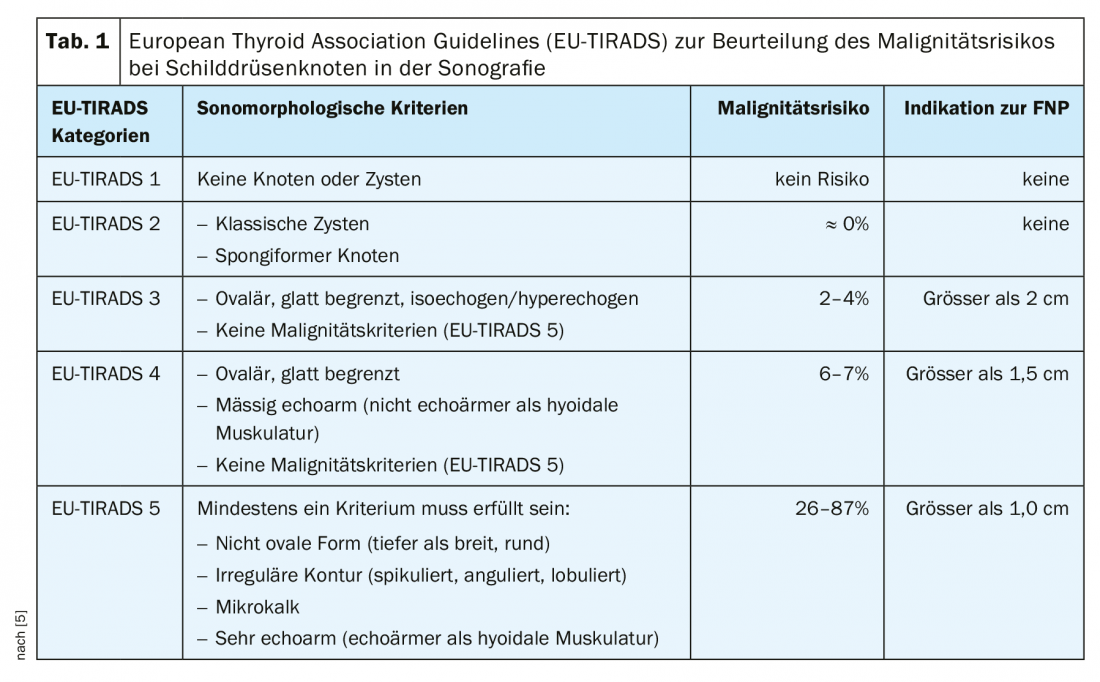
Imaging techniques: The primary examination method in the workup of a mass in the neck is ultrasound. This enables a high-resolution assessment of thyroid nodules and very precise localization diagnostics. It is advisable to use a classification system to evaluate the thyroid nodules examined sonographically. The objective of such a classification system is to provide a quantitative grading of thyroid nodules with resulting recommendations, standardized nomenclature, and a standardized report. In our latitudes, the American [7] or European [5] TI-RADS (Thyroid Imaging-Reporting and Database System) is commonly used (Fig. 2, Table 1 [5]).
In addition to the thyroid gland, the cervical lymph nodes and the other soft tissues of the neck should always be sonographically evaluated. To some extent, the neck soft tissues can also be assessed for infiltration in infiltrating thyroid carcinomas.
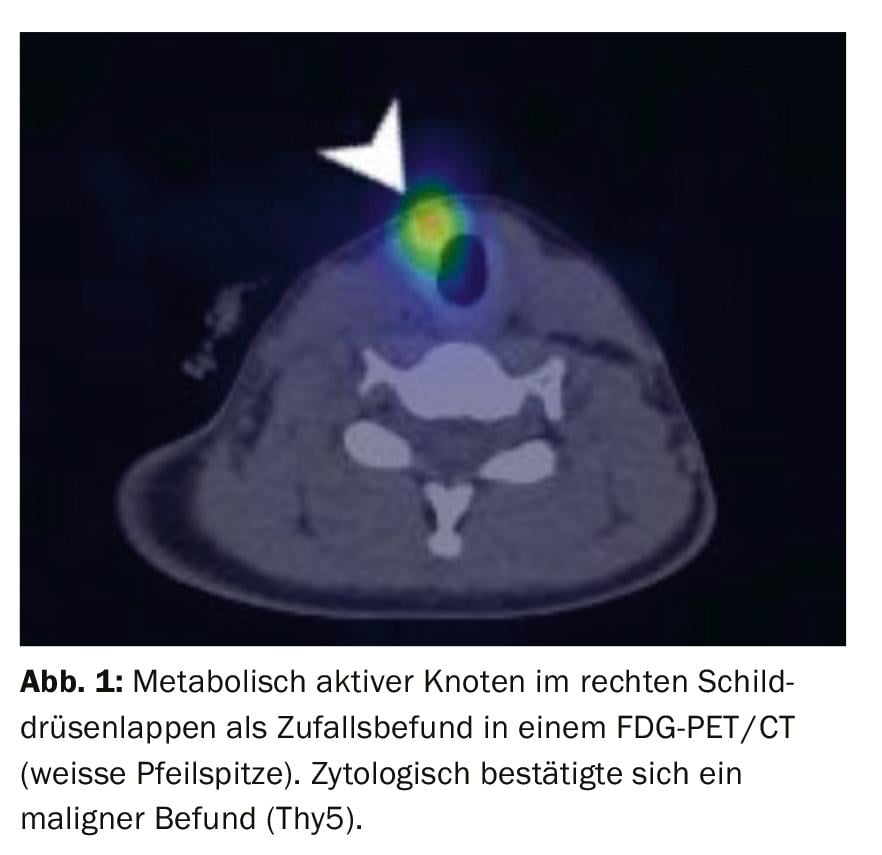
CT and MRI have limited value in the primary diagnosis of thyroid nodules. When sonography reaches its physical limit, the assessment of the anatomic location of large thyroid glands extending mediastinally and retrotracheally can be better evaluated than with ultrasound. Incidentally detected FDG-avid thyroid nodules on PET-CT should be punctured. The malignancy potential of such nodules is approximately 14-56% [6] (Fig. 1).
Fine needle aspiration (FNP) and cytological diagnosis: Fine needle aspiration is of great importance for diagnosis and determination of further procedure. The processed cells are evaluated according to either an American 6-level (Bethesda classification) or a 5-level British classification (Thy classification). From a Thy3 finding, carcinoma is no longer excluded. The risk of carcinoma here is 15-40%, with a Thy4 60-70%, and with a Thy5 97-100%.
Various mutations and translocations are now used in the diagnosis of thyroid carcinoma and will become increasingly important in the future. Currently, the most important mutations are RAS, B-RAF and hTERT. Depending on the size of the focal lesion and the cytological diagnosis, risk stratification regarding therapy and follow-up is performed.
Therapy of thyroid carcinoma
Surgery: The primary therapy for thyroid carcinoma is surgery. If the tumor is completely removed after histological workup, the patient is considered cured. This is the case when the tumor measures smaller than 2.0 cm in the pathological specimen after hemi- or total thyroidectomy, has been completely removed, and shows classical differentiation.
In all other cases, total thyroidectomy under recurrent monitoring is indicated, supplemented by ipsilateral neck dissection, usually level III-IV and level VI, depending on lymph node status. Intraoperative and postoperative risks are low in experienced surgeons; nonreversible hypoparathyroidism and laryngeal nerve lesion are considered the main risks. Depending on histology, TNM status, and increasingly genetic analysis, the appropriate postoperative treatment and follow-up regimen is determined.
Radioiodine ablation: Both the ATA [2] (American Thyroid Association) and the ETA [4] (European Thyroid Association) have developed guidelines for the indication of postoperative radioiodine therapy. Depending on histology, size of primary tumor, multifocality, lymph node number/size, and genetics, patients are classified as “low risk,” “intermediate risk,” or “high risk” for ATA and “very low,” “low,” and “high risk” for ETA. There is agreement in “very low” and “low risk” patients, where the overall risk of recurrence within 10 years is less than 3%, with or without radioiodine ablation. There is also agreement among “high risk” patients that radioiodine therapy significantly reduces the risk of recurrence over the 10-year course. The big disagreement is with “intermediate risk” patients. The ATA suggests radioiodine ablation only in selected patients, whereas the European guidelines do not list “intermediate risk” patients and recommend a more aggressive procedure overall. With the help of this risk stratification, the indication for a possible, supplementary radioiodine ablation is made, the examination intervals in the follow-up are determined and the post-therapeutic target TSH is set.
The goal of adjunctive radioiodine therapy is to find and eliminate thyroid or tumor cells left behind after surgery, thus simplifying follow-up by means of sonography of the neck and thyroglobulin measurements. The naturally occurring isotope 131iodineused for therapy is administered perorally in capsule form and accumulates within 24 hours almost exclusively in thyroid cells and well-differentiated thyroid carcinoma cells and is metabolized in these cells. The prerequisite for this is the stimulation of the cells by means of an increase in TSH, whether endogenously by thyroid hormone depletion or exogenously by means of rhTSH (Thyrogen). Which method is chosen depends on the initial risk stratification. Cells that absorb 131iodineare destroyed and broken down by the body. Thus, residual findings in the thyroid lodge, lymph node metastases as well as distant metastases can be successfully treated. The patient is considered tumor-free if there are no iodavid findings six months after surgery or radioiodine ablation and the tumor marker is not measurable under stimulation (usually by rhTSH).
All patients are substituted with thyroid hormones (levothyroxine) postoperatively. In “very low” (ETA only), “low”, “intermediate risk” (ATA only) patients a TSH of 0.5-2.0 mU/l, in “high risk” patients between 0.1-0.5 mU/l is targeted.
Treatment of residual findings (persistent disease), metastases, and recurrences: If iodavid distant metastases (most commonly lung or bone metastases) appear on whole-body scintigram under radioiodine ablation, a second radioiodine therapy is performed after exclusion of non-iodavid metastases by FDG-PET/CT or FDG-PET/MRI, usually 4-6 months after radioiodine ablation. The effect of therapy can be measured in subsequent whole-body scintigraphy and in the blood by determining thyroglobulin. With a good response, iodavid metastases are completely regressed and thyroglobulin decreases until it is no longer measurable after 3-6 months (Figs. 3-5).
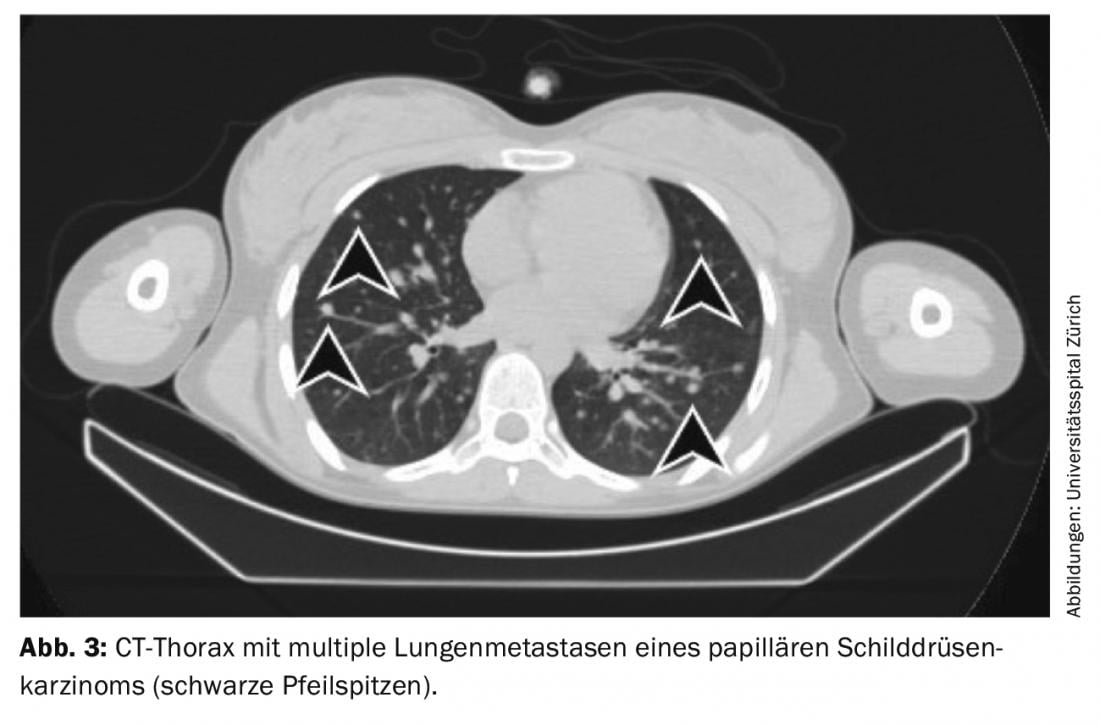
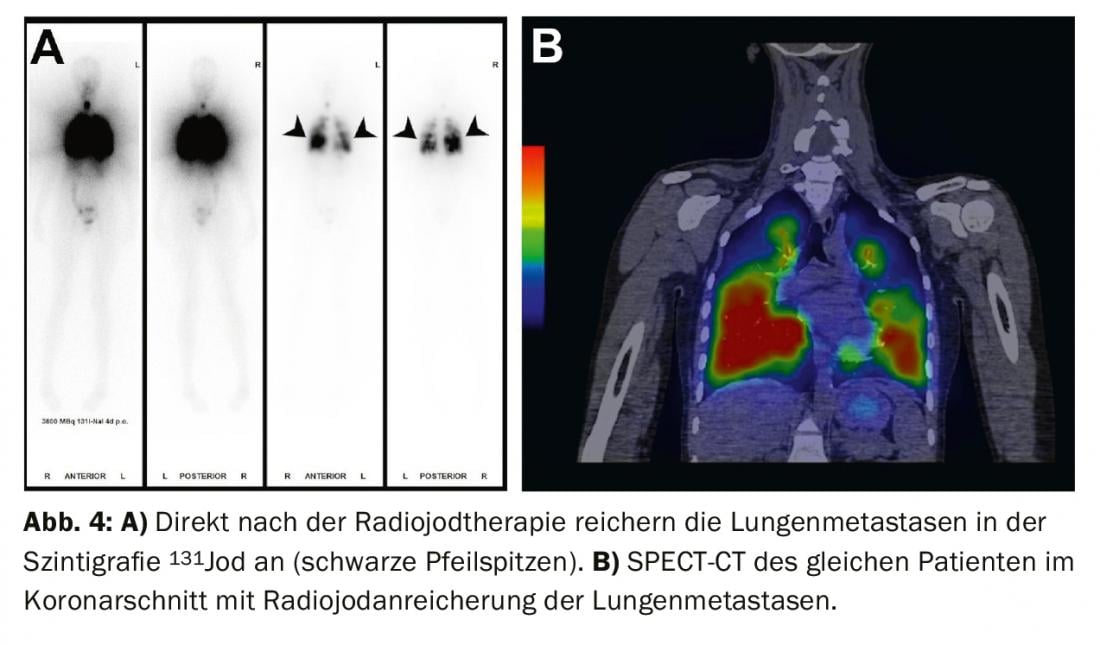
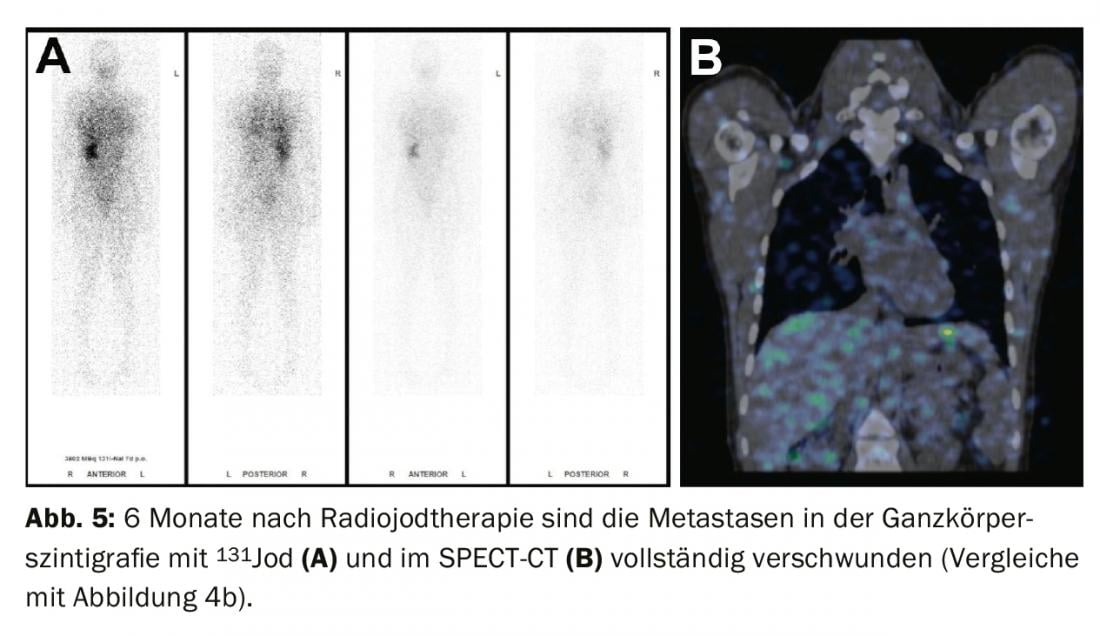
If thyroglobulin rises in the course of tumor follow-up or suspicious lymph nodes appear on neck ultrasound, the diagnosis should first be confirmed cytologically. Subsequently, radioiodine therapy is recommended with the aim of treating the metastases and completing or adjusting the staging.
Repeat radioiodine therapy can be performed as long as the metastases store iodine and a decrease in thyroglobulin can be documented. However, with each radioiodine therapy, the risk of developing radioiodine-refractory tumor cells increases. In addition, from about 22 GBq of cumulative focal dose (equivalent to about 4-5 therapies), the risk of developing a second tumor increases. Leukemias are the most common. The latency period is approximately 10 years. Radioiodine therapy is contraindicated if the metastases de-differentiate, i.e., no longer store iodine or thyroglobulin does not decrease.
Radio refractory thyroid carcinomas: Radio iodine refractory thyroid carcinomas are rare. The incidence is estimated to be about 4 to 5 cases per one million population. It mainly affects elderly patients with extensive metastasis, poorly differentiated carcinomas, and highly FDG-avid tumor manifestations. The 10-year survival rate is only 10% for radioiodine-refractory thyroid carcinomas [6].
Therapy options and management of iodine-refractory carcinomas: In radioiodine-refractory thyroid carcinomas, suppression of serum TSH is of great importance. Recommended in such situations is levothyroxine substitution with a target TSH of <0.1 mU/l. Therapeutic options include systemic therapy with tyrosine kinase inhibitors or percutaneous radiotherapy.
Radiotherapy: percutaneous radiotherapy may be used for singular osseous metastases, locally cervically when there is disseminated metastasis without a morphologic correlate on imaging, or the patient is inoperable.
Classical chemotherapy: Classical chemotherapy is less established in the treatment of thyroid carcinoma. Thus, treatments with doxorubicin show a response of 0 to 20% with high toxicity. Combination therapies with doxorubicin and cisplatin are also not promising.
Molecular target therapy – tyrosine kinase inhibitors [6]: Systemic therapy with tyrosine kinase inhibitors is most established in iodine-refractory thyroid carcinoma. The indication for a system therapy depends on various factors. The rate of tumor progression can be evaluated with thyroglobulin doubling time. However, imaging should always be used to confirm anatomic tumor progression using RECIST (Response Evaluation Criteria in Solid Tumors). Progression is defined as an increase in size of the target lesions by more than 20% or by more than 5 mm, marked size progression of the nontarget lesions, or newly appeared lesions.
In patients with multiple metastases >1-2 cm and RECIST progression within 12 months, systemic therapy is recommended. In contrast, for few and small metastases <1 cm without RECIST progression within 12 months, no system therapy is recommended. Instead, a wait-and-see approach can be adopted with regular imaging and laboratory monitoring (“active surveillance”).
Tyrosine kinase inhibitors are mainly anti-angiogenic inhibitors, and result in tumor cell growth inhibition. Progression-free survival versus placebo was 18.3 versus 3.6 months for lenvatinib, 10.8 versus 5.8 months for sorafenib, and 11.1 versus 5.9 months for vandetanib [6].
Tumor response is observed mainly in lymph node metastases, liver and lung metastases. Bone metastases show poorer tumor response. Frequently, progression of bone metastases is found during therapy with tyrosine kinase inhibitors, despite response of the remaining organ or lymph node metastases. In these cases, additional administration of bisphosphonates or denosumab may be discussed. In two-thirds of cases, complications from bone metastases develop within one year.
The optimal duration of therapy is not known. Often, therapy is maintained until side effects (diarrhea, fatigue, skin toxicity, hypertension, increased need for thyroxine, liver toxicity, hemorrhage, thrombosis, gastrointestinal fistula formation, heart failure) become prevalent or tumor progression is observed. It is unclear whether treatment should be continued in cases of only mild tumor progression or concurrent tumor response and progression. Patients with longer interruptions between therapy cycles show an increased risk of accelerated tumor progression.
Take-Home Messages
- Patients with well-differentiated thyroid carcinomas usually have a very good prognosis.
- A good preoperative assessment serves to optimize surgical planning.
- Postoperative risk stratification should take place at a center with a dedicated thyroid board.
- Postoperative therapy and follow-up should be performed at a thyroid center in patients with an “intermediate risk” situation or higher.
Literature:
- ACS. American Cancer Society [Internet]. 2019 [cited 2019 Sep 23];Available from: www.cancer.org/cancer/thyroid-cancer/about/key-statistics.html
- Haugen BR, Alexander EK, Bible KC, et al: 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; doi:10.1089/thy.2015.0020
- NICER. National Institute for Cancer Epidemiology and Registration [Internet]. 2019 [cited 2019 Sep 23]; Available from: www.nicer.org
- Pacini F, Schlumberger M, Dralle H, et al: European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006; doi:10.1530/eje.1.02158
- Russ G, Bonnema SJ, Erdogan MF, et al: European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017; doi:10.1159/000478927
- Schlumberger M, Pacini F, Tuttel RM: Thyroid Tumors. 4th ed. tcgraphite; 2015.
- Tessler FN, Middleton WD, Grant EG, et al: ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017; doi:10.1016/j.jacr.2017.01.046
InFo ONCOLOGY & HEMATOLOGY 2019; 7(5): 6-10.