CML patients have a normal life expectancy when treated with Abl-specific kinase inhibitors (TKIs) in chronic phase. Imatinib, dasatinib and nilotinib are approved in the first-line treatment of CML. In case of resistance or intolerance, bosutinib and ponatinib are available as TKI alternatives. The goal of therapy is to achieve deep molecular remission (MR). This is a prerequisite for TKI discontinuation concepts and the achievement of therapy-free remission (TFR). The biological mechanisms underlying TFR are not well understood, but are probably immunologically mediated. It is possible that temporary interferon alpha (IFN) therapy or immune checkpoint inhibitor therapy may increase the rate of TFR. These questions are currently being addressed in clinical trials.
Every patient with CML should be treated with an Abl-specific TKI approved in first-line therapy. A timely molecular therapeutic response is prognostically relevant. To assess this, regular recording of BCR-ABL mRNA load (every three months until stable MMR is achieved, then at least every six months) is essential during TKI therapy [1]. BCR-ABL copy number is measured in standardized laboratories and standardized in the international unit “IS” [2]. BCR-ABL load after IS makes a statement about the depth of remission achieved. The decrease in BCR-ABL copy number in peripheral blood is expressed in logarithmic steps from baseline at diagnosis. Simplified, an MR3 (MMR) corresponds to a decrease in BCR-ABL load by three log levels, an MR4 corresponds to a decrease by four log levels, etc.
Measurement of BCR-ABL load in relation to the timing of therapy allows evaluation of response and, if necessary, switching of therapy in case of resistance to therapy [1]. In addition to first-line therapeutics, Abl inhibitors such as bosutinib or ponatinib are available as therapeutic alternatives in cases of resistance, comorbidities or intolerance.
Allogeneic stem cell transplantation (allo-SCT) is a treatment option generally indicated only in patients with advanced CML or CML refractory to multiple TKIs.
Interferon alpha (IFN)
IFN monotherapy is poorly effective in CML patients.
In contrast, combination therapy of imatinib or a second-generation TKI (nilotinib, dasatinib) with pegylated interferon alpha (IFN) is highly effective. Deep MR is achieved more rapidly and in significantly more patients with the combination than with TKI alone [3–5]. In uncontrolled studies, IFN maintenance therapy after preceding combination therapy also induced a high rate of TFR [6,7].
Therapy goals in chronic phase
The primary goal of TKI therapy in CML is to achieve molecular remission as soon as possible, which should be at least in the range of MMR (MR3). This is important because deep molecular remission protects against disease progression and normalizes overall survival with CML [8–10].
The secondary therapeutic goal is the rapid achievement of deep MR (e.g., in terms of MR4, MR4.5, or MR5), because this allows inclusion in TKI discontinuation regimens.
Treatment free remission (“TFR”)
Originally, TKI therapy for CML was designed to be continuous, as it quickly became clear that BCR-ABL inhibitors (imatinib, dasatinib, nilotinib) could not eradicate CML stem cells [11,12].
Surprisingly, subsequent clinical case reports and studies (STIM, STIM-2, EURO-SKI) showed that about 50% of patients who have been treated with imatinib for many years and thereby achieved stable MR4.5 can pause imatinib without suffering a molecular relapse (rebound of BCR-ABL load or loss of an MMR) [13–17].
To date, the mechanisms underlying a TFR are poorly understood. Therefore, validated therapeutic strategies to increase the TFR rate are currently not established. However, immunological control of residual CML cells by T cells and NK cells is assumed. Factors associated with a significantly higher TFR rate were duration of imatinib pre-therapy (more than 5.8 years) and duration of MR4 (more than 3.1 years). The number of mature plasmacytoid dendritic cells (CD86+pDC) and mature NK cells is a potential biological marker associated with a higher rate of TFR [18,19].
Concepts for improving the TFR rate
Achieving treatment-free remission in as many patients as possible is the ambitious goal of current first-line CML therapy concepts (CML-V, Tiger) and discontinuation studies (NAUT, ENDURE, INCEPTION).
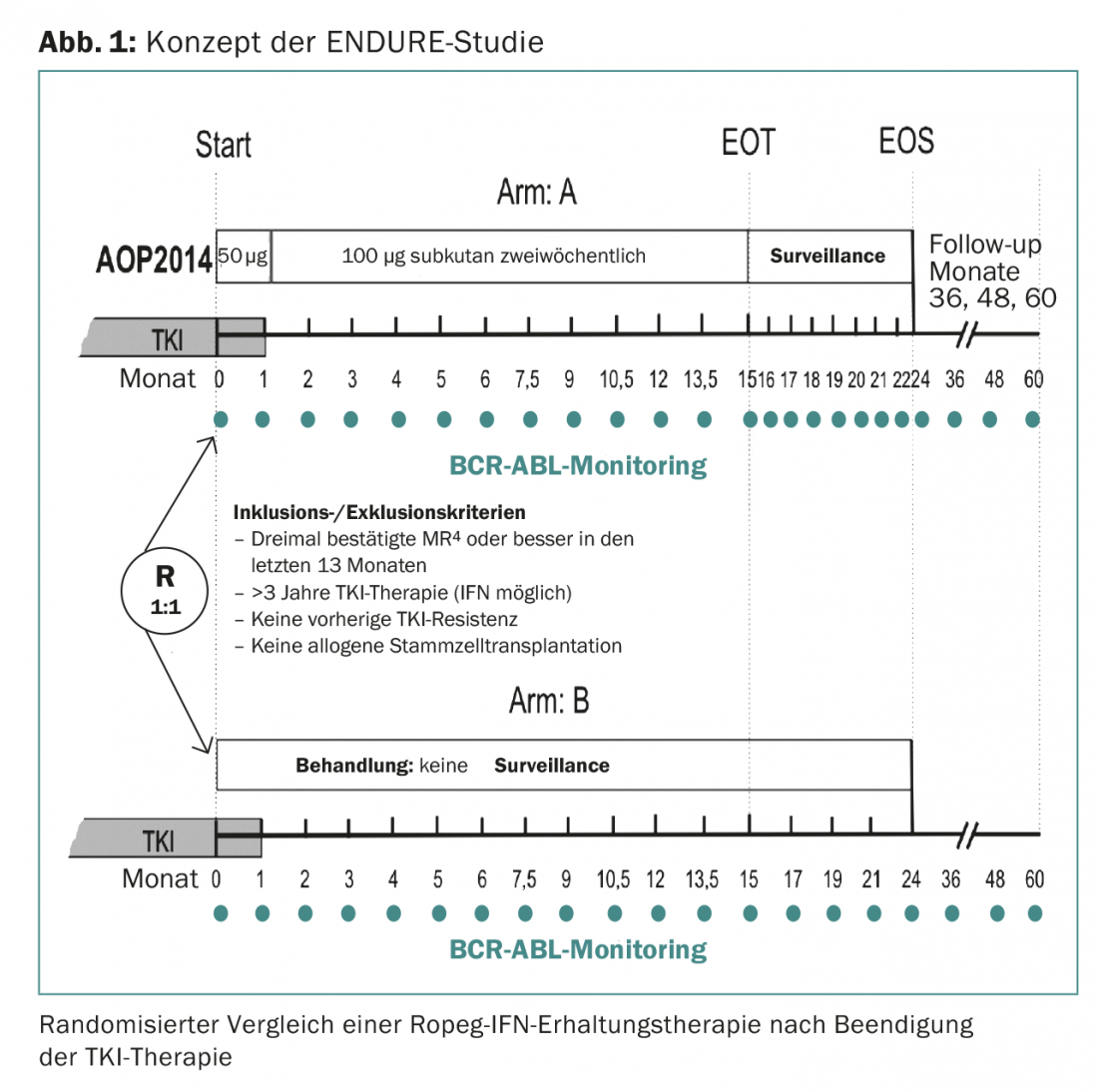
The ENDURE study, which will start shortly at 15 centers in Germany, will test whether 15 months of IFN maintenance therapy can reduce the rate of molecular recurrence in 214 patients undergoing deep MR (at least MR4) (Fig. 1). The IFN used is Ropeg-IFN (AOP2014). Ropeg-IFN is a new IFN with a longer half-life and therefore better tolerated than previously available pegylated IFN. The preparation only needs to be injected every 14 days.
Another innovative therapy concept will soon be tested in the INCEPTION study. Here, patients after TKI pausing – stratified by exhaustion T-cell phenotype and pDC immunophenotype – are randomized to the checkpoint inhibitors nivolumab and ipilimumab.
Outlook
With the introduction of TKIs as standard therapy for CML, survival with CML has normalized. The goal of future CML therapy is to achieve deep MR and long-term, safe treatment freedom in as many patients as possible.
Literature:
- Baccarani M, et al: European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013; 122: 872-884.
- Cross NCP, et al: Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 2015; 29: 999-1003.
- Simonsson B, et al: Combination of pegylated IFN-α2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood 2011; 118: 3228-3235.
- Preudhomme C, et al: Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med 2010; 363: 2511-2521.
- Nicolini FE, et al: Nilotinib and peginterferon alfa-2a for newly diagnosed chronic-phase chronic myeloid leukaemia (NiloPeg): a multicentre, non-randomised, open-label phase 2 study. Lancet Haematology 2015; 2: e37-e46.
- Burchert A, et al: Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol 2010; 28: 1429-1435.
- Burchert A, et al: Interferon alpha 2 (IFN) maintenance therapy may enable high rates of treatment discontinuation in chronic myeloid leukemia (CML). Leukemia 2015; 29: 1331-1335.
- Hehlmann R, et al: Deep Molecular Response Is Reached by the Majority of Patients Treated With Imatinib, Predicts Survival, and Is Achieved More Quickly by Optimized High-Dose Imatinib: Results From the Randomized CML-Study IV. J Clin Oncol 2014 Feb 10; 32(5): 415-423.
- Hehlmann R, et al: Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-α in newly diagnosed chronic myeloid leukemia. J Clin Oncol 2011; 29: 1634-1642.
- Bower H, et al: Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol 2016; 34: 2851-2857.
- Graham SM, et al: Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002; 99: 319-325.
- Jørgensen HG, et al: Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood 2007; 109: 4016-4019.
- Mahon FX, et al: Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010; 11: 1029-1035.
- Rousselot P, et al: Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 2007; 109: 58-60.
- Rousselot P, et al: Loss of Major Molecular Response As a Trigger for Restarting Tyrosine Kinase Inhibitor Therapy in Patients With Chronic-Phase Chronic Myelogenous Leukemia Who Have Stopped Imatinib After Durable Undetectable Disease. J Clin Oncol 2013; 32: 424-430.
- Ross DM, et al: Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 2013; 122: 515-522.
- Mahon FX, et al: Interim Analysis of a Pan European Stop Tyrosine Kinase Inhibitor Trial in Chronic Myeloid Leukemia: The EURO-SKI study. Blood 2014; 124: 151.
- Ilander M, et al: Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia 2016. DOI: 10.1038/leu.2016.360 [Epub ahead of print].
- Schütz C, et al: Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia 2017. DOI: 10.1038/leu.2017.9 [Epub ahead of print].
InFo ONCOLOGY & HEMATOLOGY 2017; 5(1): 17-19.