The prevalence of ulcerative colitis (CU) in Switzerland is 1/500. The JAK inhibitor tofacitinib has been an arrow in the treatment quiver since 2019. In the phase 3 OCTAVE study, it proved to be effective in achieving and maintaining clinical and endoscopic remission compared to placebo. In the real world, however, the data situation in Switzerland has so far been inadequate. A study group has now addressed this issue.
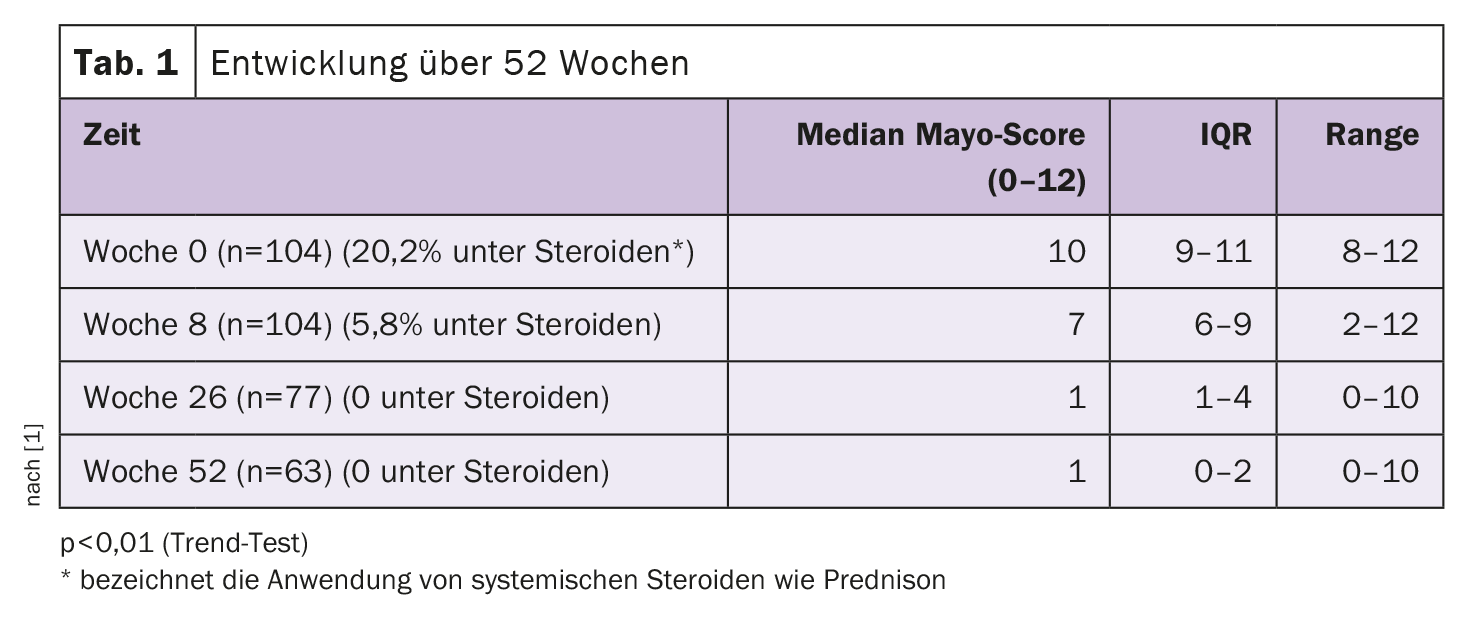
The aim of the post-marketing study FACES (First Tofacitinib Experience in Switzerland) was to assess the efficacy and safety of tofacitinib in the treatment of adults with moderate to severe ulcerative colitis in Switzerland under real-world conditions. In accordance with the OCTAVE study design, the research group assessed clinical and endoscopic activity at baseline and at weeks 8, 26 and 52 using the Mayo score, as explained by Prof. Dr. Alain Schoepfer, Department of Gastroenterology and Hepatology, Lausanne University Hospital, and member of the FACES survey group [1]. Tofacitinib was administered 2× 10 mg per day during the 8-week induction phase and 2× 5 mg during the maintenance phase.
In the Mayo scoring system, the factors stool frequency, rectal bleeding, endoscopy findings and the physician’s overall assessment are taken into account to evaluate the activity of CU. Each of these four components is rated individually from 0 to 3, resulting in a total score of 0 to 12.
Significant decline in Mayo score
The researchers included 104 adults in their study (mean age 41 ± 14.4 years, median duration of illness 6 years, IQR 3- 11, range 1-44 years, 48.1% women). The patients’ diseases were localized:
- Proctitis 3.9%,
- left-sided colitis 34.6%,
- extensive colitis 11.5%,
- Pancreatitis 50%.
All participants had received mesalazine, topical budesonide and prednisone during pre-treatment, 70.2% also received azathioprine. Before starting tofacitinib therapy, the patients had undergone a median of 2 unsuccessful biologic treatments (mainly infliximab and vedolizumab (79% and 62% respectively), as well as golimumab (23%), adalimumab, ustekinumab (20% each) and tacrolimus (15%); IQR 1-3, range 0-6). 21 subjects (20.2%) were also given prednisone at the start of treatment with tofacitinib. Over time, a significant decrease in the Mayo score was observed (Table 1) .
At week 8, adherence to tofacitinib was still 100%. After 26 weeks, 77 of the 104 patients (74%) remained adherent; after one year, 63/104 (60.6%) were still adherent. No deep vein thrombosis, pulmonary embolism, serious advanced cardiovascular events or neoplasia were observed. Herpes zoster reactivation occurred in four patients during the induction phase and in two during the maintenance phase.
In terms of stool frequency, all patients had at least three more bowel movements than normal at baseline. After 52 weeks, this could be reduced to a normal level or a maximum of 1-2 bowel movements more in almost all patients (Fig. 1).
In this Swiss CU population, which was characterized by a complicated disease course and multiple failures of biologic therapies, more than 60% were still on tofacitinib at week 52 as they continued to have clinical benefit, Prof. Schoepfer concluded. With a favorable safety profile, no patient was treated simultaneously with systemic steroids in weeks 26 and 52.
Congress: SGG Annual Congress 2023
Source:
- Schoepfer A: Presentation “Real-life efficacy and safety of tofacitinib to treat moderate-to-severe ulcerative colitis in Switzerland”; Annual Congress of the Swiss Society of Gastroenterology (SGG), Interlaken, 14.09.2023.
GASTROENTEROLOGY PRACTICE 2023: 1(2): 31 (published 27.11.23, ahead of print)