New Phase III data from the DISCOVER-1 and DISCOVER-2 studies show improvement in joint and skin symptoms of psoriatic arthritis at week 52, and an indication expansion of guselkumab is currently under review. It would be another advance in treatment options for this burdensome autoimmune disease.
Data from the two studies in the DISCOVER program formed the basis for the regulatory submission of Tremfya® for the treatment of adult patients with active PsA. Approximately 75% of patients treated with Tremfya® achieved an ACR-20 response in the DISCOVER-1 and DISCOVER-2 studies [1,2]. These are the first 1-year Phase III results evaluating specific IL23p19 inhibition in active psoriatic arthritis. The data were presented at the European League Against Rheumatism (EULAR) E-Congress 2020 and published in The Lancet [3–5]. In the two Phase III clinical trials, guselkumab (Tremfya®) demonstrated improvements in adult patients with active psoriatic arthritis (PsA) across multiple clinical endpoints, including joint and skin symptoms, soft tissue inflammation, physical functioning, and reduction in radiographic progression at week 52 [1,2]. Tremfya® is not currently approved for the treatment of PsA and is currently under review by Swissmedic for this use.
| Abbreviations ACR = ACR responder criterion MDA = Minimal Disease Activity DAS-28 = Disease Activity Score 28 VLDA = Very Low Disease Activity HAQ-DI = Health Assessment Questionnaire Disability Index SF-36 = Short Form 36 Health Survey Questionnaire PCS = Physical Component Summary MCS = Mental Component Summary |
DISCOVER-1 and DISCOVER-2 demonstrate efficacy and safety
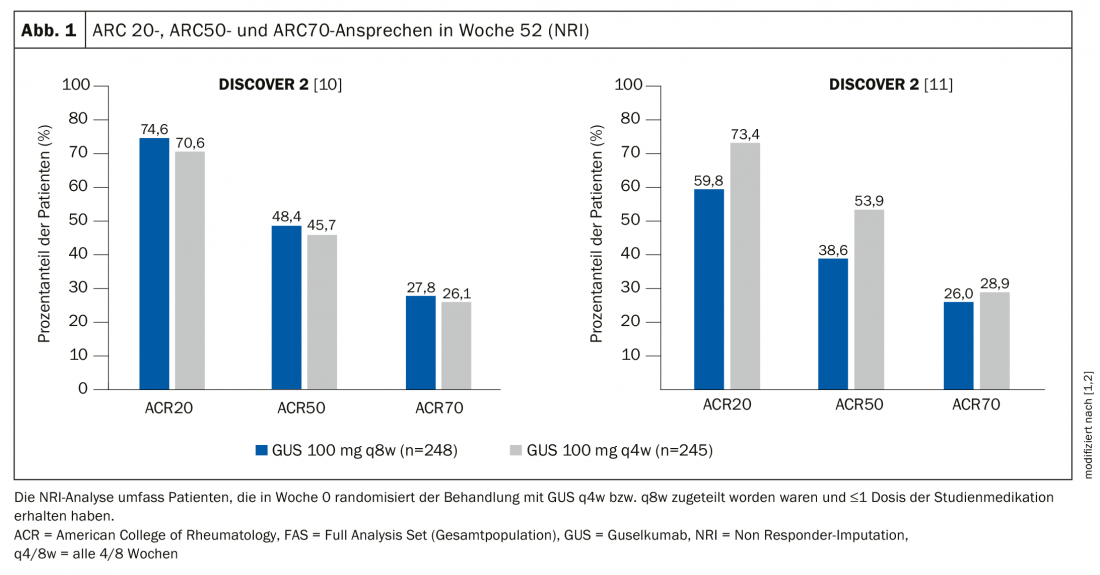
Patients included in DISCOVER-1 were either biologics-naïve or previously receiving anti-TNF-α biologics. DISCOVER-2 included only biologics-naive patients. DISCOVER-2 also examined radiographic progression of joint damage. In both studies, patients were randomized over a one-year period to two verum groups, Tremfya® 100 mg every 4 weeks (q4w) or every 8 weeks (q8w), and placebo groups with crossover at week 24 to Tremfya® q4w. The results were presented as a poster at this year’s EULAR e-congress [1,2]. The DISCOVER trials similarly demonstrated improvements in several secondary endpoints at week 52 compared with week 24, including ACR70 response, resolution of soft tissue inflammation (enthesitis and dactylitis) [7], disease activity score (DAS-28), C-reactive protein (CRP) [4], minimal disease activity (MDA) [8], very low disease activity (VLDA) [9], improvement in physical functioning (HAQ-DI) [7], overall improvement in health status (SF-36*, PCS**, MCS†).
In both studies, Tremfya® was well tolerated until study completion, and the adverse events observed were generally consistent with previous studies of Tremfya® and current prescribing information [6]. Serious adverse effects and serious infections occurred in 4% and 1% of Tremfya® treated patients, respectively, in both DISCOVER-1 and DISCOVER-2. There were no reported deaths in Tremfya® treated patients, and no Tremfya treated patient had inflammatory bowel disease, opportunistic infections, active tuberculosis, or anaphylactic or serum sickness-like reactions [1,2].
Source: Janssen
*/** SF-36 is a patient questionnaire that measures functional health and well-being based on patient-reported outcomes. As part of SF-36, the PCS subscale is composed of four scales that assess physical function, limitations caused by physical problems, somatic pain symptoms, and general health.
† MCS is composed of four scales that assess vitality, emotional impact, social functioning, and mental health.
Literature:
- Ritchlin C, et al: Guselkumab, an IL-23 Inhibitor That Specifically Binds to the IL23p19 Subunit, for Active Psoriatic Arthritis: One Year Results of a Phase 3, Randomized, Double-blind, Placebo-controlled Study of Patients Who Were Biologic-Naïve or TNFα Inhibitor-Experienced. SAT0397. Presented at the 2020 EULAR E-Congress June 3-6.
- McInnes I, et al: Efficacy and Safety of Guselkumab, a Monoclonal Antibody Specific to the p19-Subunit of Interleukin-23, Through Week 52 of a Phase 3, Randomized, Double-blind, Placebo-controlled Study Conducted in Biologic-naïve Patients with Active Psoriatic Arthritis. SAT0402. Presented at the 2020 EULAR E-Congress June 3-6.
- Business Wire. Janssen Seeks to Expand Use of TREMFYA, www.businesswire.com/news/home/20191022006172/en/Janssen-Seeks-Expand-TREMFYAC2AEE296%BC-guselkumab-Treatment-Adults® (guselkumab) in the Treatment of Adults With Active Psoriatic Arthritis. last call 07/28/2020.
- Deodhar A, et al: Guselkumab in Patients with Active Psoriatic Arthritis who were Biologic-naive or had Previously Received TNFα Inhibitor Treatment (DISCOVER-1): a Double-blind, Randomised, Placebo-controlled Phase 3 Trial. The Lancet 2020; 395: 1115-1125.
- Mease PJ, et al: Guselkumab in Biologic-naive Patients with Active Psoriatic Arthritis (DISCOVER-2): A Double-blind, Randomised, Placebo-controlled Phase 3 Trial. The Lancet 2020; 395: 1126-1136.
- TREMFYA® Technical Information, as of 09/2019 available at www.swissmedicinfo.ch
- Clinicaltrials.gov: A Study Evaluating the Efficacy and Safety of Guselkumab Administered Subcutaneously in Participants With Active Psoriatic Arthritis Including Those Previously Treated With Biologic Anti-Tumor Necrosis Factor (TNF) Alpha Agent(s) (DISCOVER-1). Identifier: NCT03162796. https://clinicaltrials.gov/ct2/show/NCT03162796, last accessed 07/28/2020.
- Gossec L, et al: Minimal Disease Activity as a Treatment Target in Psoriatic Arthritis: A Review of the Literature. J Rheumatol 2018; 45: 6-13.
- Coates L, et al: Abstract 2548. Presented at the 2017 ACR/ARHP Annual Meeting. Available at: https://acrabstracts.org/abstract, last accessed 07/28/2020.
- McInnes, et al: Presented at the EULAR e-congress, June 2020. poster 0402.
- Ritchlin, et al: Presented at the EULAR e-congress, June 2020, poster 0397.
FAMILY PRACTICE 2020, 15(8): 36-37
DERMATOLOGY PRACTICE 2020; 30(4): 28-29