The pathogenesis of chronic spontaneous urticaria is very complex. In a subpopulation of affected patients, activation of mast cells by IgE plays a crucial role. The question of how autoallergens interact with IgE and trigger degranulation of skin mast cells is the subject of current research and was the starting point of a study involving researchers from Charité Universitätsmedizin Berlin (D).
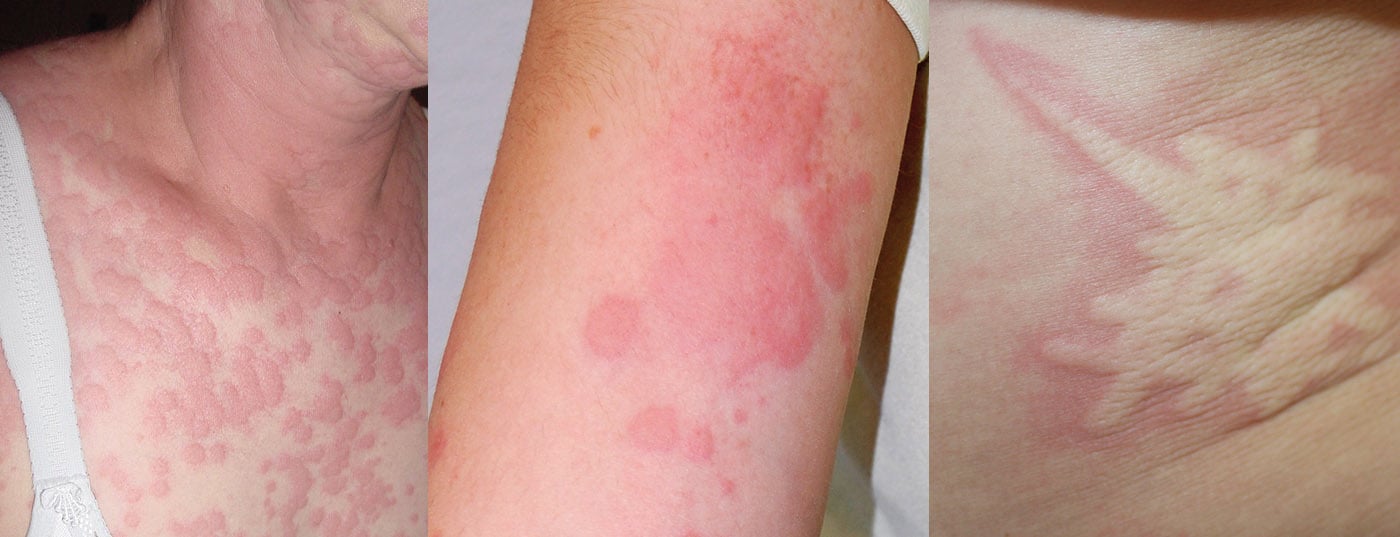
In chronic urticaria, the symptoms persist for more than six weeks and represent a considerable burden for many patients. More than half of those affected have wheals and angioedema, one-third have wheals exclusively, and about one in ten have angioedema only [1]. The disease, which occurs at any age, is divided into chronic spontaneous urticaria (csU) and chronic inducible urticaria (CIndU) [2,3]. Urticaria is a mast cell-associated disease. According to empirical findings, most cases of csU are caused by autoantibodies either against the α-chain of FcεRI or against IgE itself, thus can be considered as a form of autoimmunity [4,5]. The interaction of the autoantibody with the receptor triggers degranulation of the mast cells, resulting in a urticarial episode.

Type I vs. type IIb autoimmunity
Recently, two groups of signaling cascades have been classified: In type I autoimmunity (also called autoallergy), patients develop IgE antibodies against endogenous structures [6]. In autoimmune type IIb, IgG-type autoantibodies are directed against IgE antibodies or against the IgE receptor (FcεRI) [6,7]. csU of type I is usually associated with elevated IgE levels and a good response to omalizumab, in type IIb, total IgE is generally low [6]. The less common csU TYPE IIb can be diagnosed by laboratory diagnosis using a basophil activation test (BAT) on patient-derived serum [7]. Currently, identification of autoallergens in type I csU is only possible in specialized centers, as a variety of triggers have been described (e.g., IL-24 and TPO) [7].
What is the role of IgE autoantibodies against IL24 and against TPO?
IgE autoantibodies to TPO and to IL-24 are thought to contribute to pathogenesis in a subpopulation of csU patients. On the question of whether these IgE autoantibodies can form mast cell-activating IgE autoantigen immune complexes (auto-ICs) with their autoallergens, a study was presented at this year’s ADF Annual Meeting. Herein, the prevalence and relevance of auto-ICs in CSU were investigated [8]. Serum levels of IgE-anti-IL-24 and IgE-anti-TPO were measured in 332 CSU patients using a specific enzyme-linked immunosorbent assay ( ELISA ) and analyzed for correlations with clinical parameters and laboratory values. The researchers used the β-hexosaminidase release assay to evaluate auto-ICs for mast cell-activating effects in human skin (Box) [8]. The enzyme β-hexosaminidase released during the release of granular contents into the extracellular space correlates with the release of histamine and chymase [9,10]. Therefore, measurement of the enzymatic activity of released β-hexosaminidase provides information about the extent of mast cell degranulation [9,10].
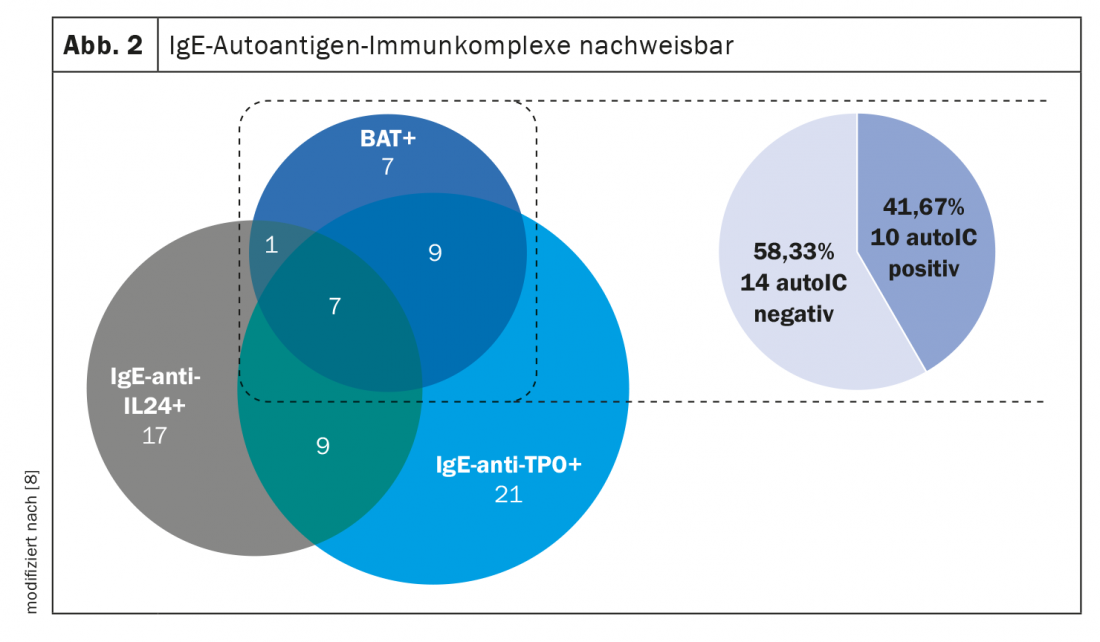
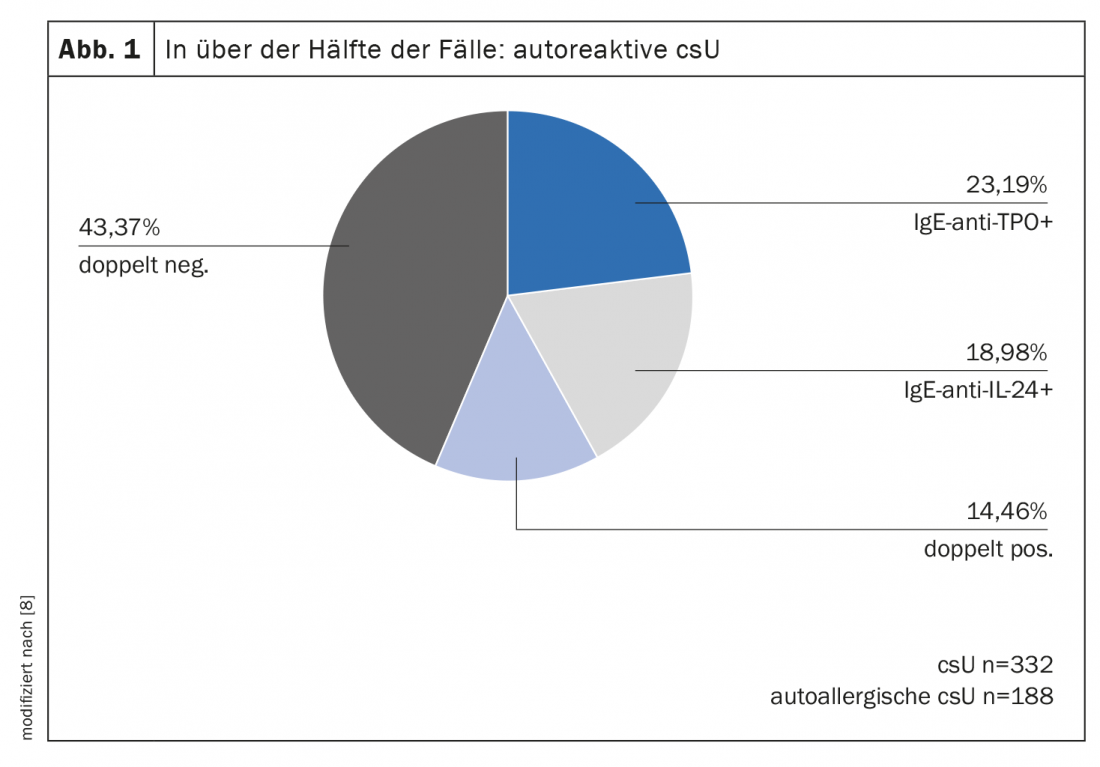
As the analysis of the study shows, with 56%, more than half of the 332 csU patients had elevated IgE-anti-IL-24 and/or IgE-anti-TPO levels (Fig. 1) [8]. Of the csU patients who tested positive in the basophil activation test, IgE autoantigen complexes were detectable in approximately 40% (Fig. 2). IgE/TPO auto-ICs were detected in 20% and IgE/IL-24 auto-ICs in 8% of the patients tested. Serum levels of free IgE-anti-TPO correlated with IgE/TPO-auto-ICs. In contrast, no correlation was shown with respect to free IgE-anti-IL-24 and IgE/IL-24 auto-IC serum levels. IgE/IL-24 auto-ICs showed a weak association with disease activity (r=0.172, p=0.007) and blood eosinophil count (r= -0.218, p<0.001). Sera from patients with high serum levels of IgE/IL-24 auto-ICs induced dose-dependent mast cell activation. In summary, approximately 50% of the CSU patients studied had autoreactive IgE. These IgE autoantibodies can form mast cell-activating immune complexes with their autoallergens. Further studies are needed to confirm the clinical relevance of these autoICs and to find out more about their impact on treatment response, the researchers said.
Congress: Working Group Dermatological Research
Literature:
- Zuberbier T, et al: The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. The 2017 Revision and Update. Allergy 2018; 73: 1393-1414.
- Bauer A, et al: Expert consensus on practical aspects in the treatment of chronic urticaria. Allergo J Int 2021; 30: 64-75.
- Maurer M, et al: The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy 2017; 72: 2005-2016.
- Murphy K, Weaver C: Allergies and allergic diseases. Janeway Immunology. 2018: 783-834.
- Lakin E, et al: On the Lipophilic Nature of Autoreactive IgE in Chronic Spontaneous Urticaria. Theranostics 2019; 9(3): 829-836.
- Maurer M, et al: Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol 2020; 181(5): 321-333.
- IMD Laboratory Berlin, Diagnostic Information No. 338, www.imd-berlin.de/fileadmin/user_upload/Diag_Info/338_Diagnostik_Urtikaria.pdf (last retrieved 11.03.2022)
- Xiang Y, et al: Prevalence and relevance of IgE-autoantigen immunocomplexes in chronic spontaneous urticaria, P007, Allergy, ADF Annual Meeting Feb 23-26, 2022.
- Ghulam O: Mast cells and proteinase-activatable receptors (PAR): expression of functional PAR by primary human mast cells and influence of PAR ligand thrombin on FcεRI -mediated degranulation response. https://macau.uni-kiel.de/servlets/MCRFileNodeServlet/dissertation_derivate_00003420/Oranos_Ghulam_Doktorarbeit.pdf, (last accessed 11.03.2022)
- Schwartz LB et al: Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J. Immunol 1979; 123: 1445-1450.
- Kocatürk E, et al: The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy 2021; 76(3): 816-830.
DERMATOLOGIE PRAXIS 2022; 32(2): 50-51