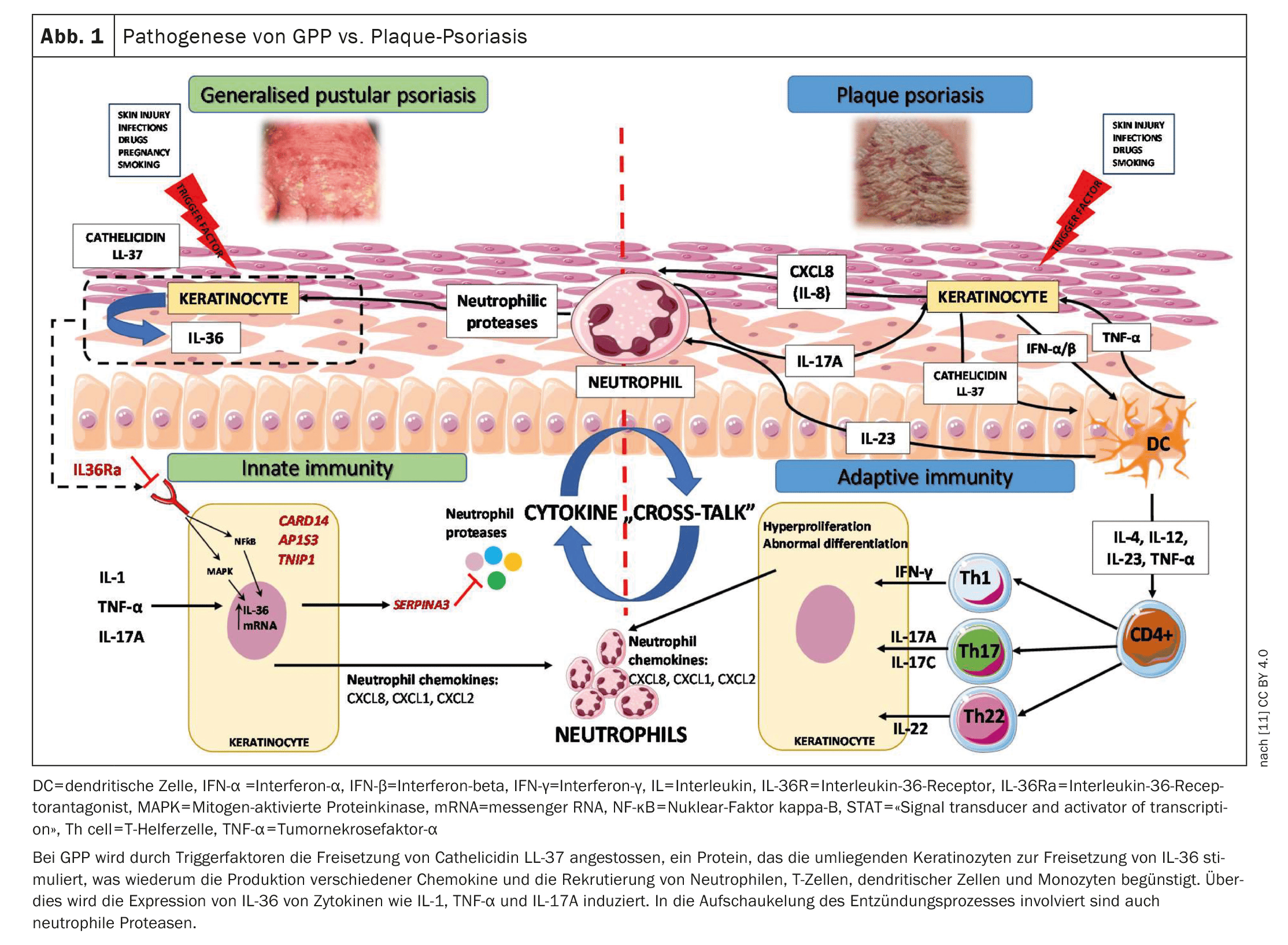
Generalized pustular psoriasis (GPP) is a neutrophilic, inflammatory skin disease with a potentially life-threatening course. Recent findings on immunopathogenesis indicate that the group of interleukin (IL)-36 cytokines are central drivers of autoinflammatory reactions in GPP. The monoclonal antibody spesolimab inhibits the activation of the IL-36 receptor (IL-36R) and has also been approved in Switzerland since last year for the treatment of adult patients with GPP.
The European Rare and Severe Psoriasis Expert Network ( ERASPEN) defines generalized pustular psoriasis (GPP) as a disease with primary, sterile, macroscopically visible pustules on non-acral skin. GPP is characterized by flare-ups with extensive pustule formation, which can be accompanied by systemic inflammatory reactions** and serious extracutaneous complications. The criteria for systemic inflammation are fever >38 °C and leukocytosis£ [1]. A GPP relapse is usually followed by phases with little or no disease activity, but there are also courses with persistent symptoms (>3 months). During an acute flare-up, symptoms such as pain, itching and fatigue lead to a high level of stress for those affected and, as a result, their quality of life is severely restricted [2–4]. Overexpression of IL-36 cytokines plays a central role in the immunopathogenesis of GPP (Fig. 1) [5,6]. The anti-IL-36 receptor antibody spesolimab blocks the IL-36 receptor (R) signaling pathway. Binding of spesolimab to IL-36R prevents the subsequent activation of IL-36R by its ligands (IL36 α, β and γ) and the downstream activation of proinflammatory signaling pathways [7]. This dampens inflammatory reactions and attenuates the symptoms of GPP [8]. Spesolimab (Spevigo®) is indicated for the treatment of relapses of GPP in adult patients [9]. The recommended dose is a single dose of 900 mg (2 vials of 450 mg each), administered as an intravenous infusion [8].
** Definition according to the American Society of Chest Physicians
£ Leukocytosis = number of white blood cells >12×109/l
63-year-old patient: acute GPP relapse and reduced general condition
The patient presented at [10] with an acute episode of previously known GPP that had been present for three days. On admission, the patient was in a reduced general condition (AZ) and showed pronounced skin findings with extensive erythematous plaques with multiple, partly confluent pustules on the entire integument. Laboratory chemistry revealed a significant increase in inflammatory parameters.
Previous therapies: Since initial diagnosis in 2011, treatment with methotrexate, dimethyl fumarate and ustekinumab. The latter was discontinued 6 months ago after several years due to an operation.
Current course of therapy: Initial systemic steroid therapy with 80 mg/d prednisolone. Topically, steroid-containing topicals were used. This led to further extensive formation of new pustules, so that after carrying out the necessary preliminary examinations, treatment with Spesolimab 900 mg i.v. was administered as a single dose. A significant reduction in disease activity was observed on the following day with a complete reduction in pustules just three days after the infusion and a rapid improvement in the AZ. Within the following week, the plaques clearly faded and the inflammation values decreased, so that the patient could be discharged from hospital ten days after the infusion. Prednisolone could be reduced to 60 mg/d at the time of discharge. In the course of the treatment, an outpatient reintroduction of ustekinumab is planned as maintenance therapy. In summary, treatment with spesolimab in this case showed a rapid improvement in the symptoms of acute GPP relapse, which is consistent with the results of clinical studies.
Congress: Dermatology compact & practical
Literature:
- Bone RC, et al: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1644-1655.
- Burden AD, et al: Symptom Experience and Content Validity of the Psoriasis Symptom Scale (PSS) in Patients with Generalized Pustular Psoriasis (GPP). Dermatol Ther (Heidelberg) 2022; 12(6): 1367-1381.
- Lebwohl M, et al: The Disease Burden of Generalized Pustular Psoriasis: Real World Evidence From CorEvitas’ Psoriasis Registry. Journal of Psoriasis and Psoriatic Arthritis 2022; 7(2): 71-78.
- Reisner DV, et al: Impact of Generalized Pustular Psoriasis from the Perspective of People Living with the Condition: Results of an Online Survey. Am J Clin Dermatol 2022; 23(Suppl 1): 65-71.
- Marrakchi S, et al: Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med 2011; 365: 620-628.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG: An update on generalized pustular psoriasis. Expert Rev Clin Immunol 2019; 15: 907-919.
- European Medicines Agency, https://ec.europa.eu/health/documents/community-register/2022/
20221209157574/anx_157574_en.pdf, (last accessed 16.05.2024) - Swissmedic: Spevigo® (active substance: spesolimab), Public Summary SwissPAR of 29.02.2024, first authorization in Switzerland: 09.08.2023
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch,(last accessed 16.05.2024)
- Kluck R, et al: Treatment of an acute flare of generalized pustular psoriasis with spesolimab, P046. JDDG 2024; 22, Issue S1: 1-40.
- Samotij D, Szczęch J, Reich A: Generalized Pustular Psoriasis: Divergence of Innate and Adaptive Immunity. International Journal of Molecular Sciences. 2021; 22(16): 9048. www.mdpi.com/1422-0067/22/16/9048,(last accessed 16.05.2024).
DERMATOLOGIE PRAXIS 2024; 34(3): 26-27 (published on 14.6.24, ahead of print)