With the Swiss MS Registry, the Swiss Multiple Sclerosis Society launched a project in 2016 that functions according to the principle of Citizen Science. On World MS Day 2018, people with MS, healthcare professionals and representatives of the MS Society took stock.
It is estimated that 2.5 million people worldwide suffer from multiple sclerosis, and approximately 700,000 in Europe [1]. But the number of unreported cases is high; the data concerning the situation in Switzerland date back to the 1990s. Now, registrations for the Swiss MS Registry (SMSR), among others, show that the number needs to be adjusted from 10,000 to 15,000 affected persons nationwide [2]. One reason so little is known about the prevalence of multiple sclerosis is that, like many diseases, MS is not reportable. Moreover, MS is particularly difficult to detect because this autoimmune disease manifests itself in so many different ways, says Prof. Milo Puhan, MD, Head of the Institute of Epidemiology, Biostatistics and Prevention at the University of Zurich (EBPI). He and his team are responsible for the analysis of the registry data.
The Swiss MS Register as a pioneering project
The Swiss MS Registry was initiated by affected members of the Swiss Multiple Sclerosis Society. In 2016, the MS Society implemented the registry as a 25-year observational study [3]. The project follows the citizen science approach that is now increasingly used: as MS experts, people affected feed their information directly into the registry, thus contributing to the formation of a broad database (box). For example, the Swiss MS Registry is primarily a collection of data that can provide information about the disease as well as the use and effect of therapies, explains Patricia Monin, director of the MS Society, in a panel discussion on the occasion of World MS Day 2018 [4]. Those affected can report on their everyday experiences and share this knowledge. This is done through surveys and diary entries that can be accessed online after registering on the MS Society platform; participation in the surveys is also possible by mail.
This new opportunity for knowledge transfer and networking is highly appreciated by the already registered study participants. “We are a knowledge community,” says Ivy Spring, who has MS, which allows people to support each other. The goal is for those affected to find each other.
The project is financed entirely by donations without the involvement of political or economic interest groups. The scientific implementation lies with the researchers of the EBPI, whose interest in knowledge is directed towards the individual experience of the persons concerned. This makes the registry a pioneering project throughout Switzerland; MS registries already exist in Germany and the United Kingdom, among other countries. The primary endpoints of the study are quality of life: how people with MS live with their disease and how they live with their condition. what does your private and professional life look like? What drug, non-drug, and alternative medicine therapies are used and to what extent are they even accessible? Secondary endpoints of the SMSR focus on symptomatology, which can vary greatly from individual to individual and depending on stage, adverse drug reactions, and the frequency of MS relapses. By involving people with MS, the initiators also hope to obtain information about the actual number of people affected [3]. The data will help improve treatments and enhance the quality of life for people with MS. They are stored in encrypted, anonymous form in the custody of the University of Zurich.

Complementing clinical studies with experiential knowledge
With its focus on the daily lives of people with MS, the Swiss MS Registry provides a complement to clinical trials such as the Swiss MS Cohort Study (SMSC). In the SMSC, 960 MS patients have been regularly screened since 2012. Every six or twelve months, there is an interview, a blood draw, a neurological examination and, if medically indicated, a standardized MRI test. The aim is to study 1000-2000 patients over a period of ten years in order to investigate the long-term effect and safety of drug therapies [5,6]. Data from the SMSC as well as from medical records (with patient consent) are again incorporated into the registry. Both the SMSR and SMSC establish P4 medicine: preventive, prospective, personalized, and participatory disease management. The participation of people with MS is especially important where so-called soft symptoms, such as fatigue, need to be recorded and treated. For Prof. Pasquale Calabrese, MD, Head of the Department of Cognitive Neuroscience at the University of Basel, quality of life is more than just a physical state; it also has social, spiritual, psychological and cognitive dimensions. “These are all things that are treated secondarily in classical clinical trials. The fact that we are bringing this to the fore here [im SMSR] is what is so revolutionary about it.”
The output of the MS register
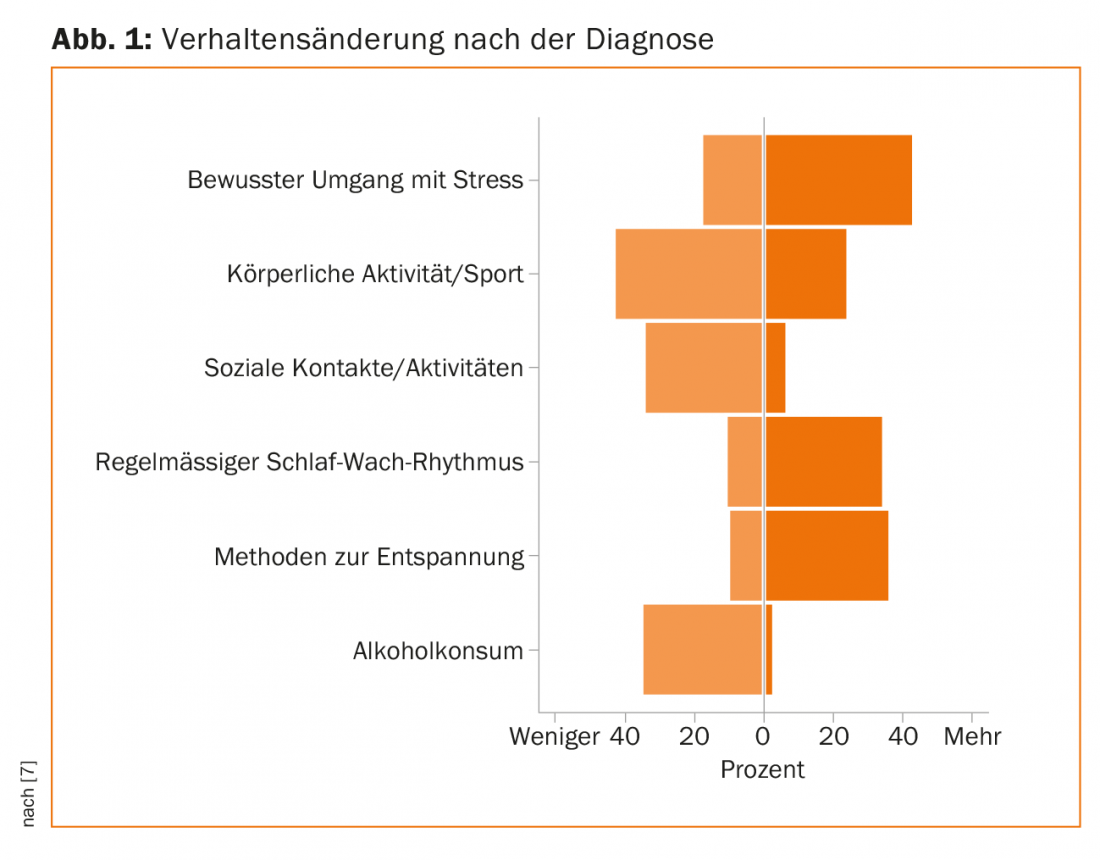
To capture the multidimensional construct of quality of life, EBPI staff use questionnaires that study participants can complete online or by mail. Each month, evaluated data are made available to interested parties via the MS Society’s homepage in the form of a “monthly graph”. In March, the MS Society published a presentation on “Behavior change after diagnosis” (Fig. 1). Striking is a reduction of physical activities as well as an abstention from alcohol in 40% of the respondents. A decrease is also evident in the area of social contacts. On the other hand, more attention is being paid to better stress management, relaxation techniques and good sleep hygiene.
Various experts are involved in the research process: Patients, specialists, basic researchers, neurologists, psychologists, nurses and physiotherapists. Further development of the MS Registry is also driven in collaboration with people affected by the disease; study participants can provide input at the annual resonance group meetings, which bring together subject matter experts, MS Society members, and people affected by the disease.
Dr. Christoph Lotter, Vice Director of the MS Society, is convinced that the Swiss MS Registry will help to develop an understanding of the real needs of people with MS and to better allocate resources. The president of the Zurich cantonal government and health director Dr. Thomas Heiniger agrees: “The MS Registry offers a unique opportunity for people with MS to contribute their personal views and needs and thus help to ensure that the issues that are really relevant to them are also clarified. According to Prof. Dr. med. Andrew Chan (Neurozentrum Inselspital Bern), the fact that the number of people affected has already had to be corrected upwards by half after two years of data collection will have a major impact, for example with regard to access to and the costs of medication or the number of rehabilitation and physiotherapy places.
Literature:
- Browne P, et al: Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014; 83(11): 1022-1024.
- Swiss Multiple Sclerosis Society: Annual Report 2017. Swiss Multiple Sclerosis Society in Facts and Figures.
- Swiss Multiple Sclerosis Registry (SMSR). 2016. https://clinicaltrials.gov/ct2/show/NCT02980640
- Discussion panel on the Swiss MS Registry. May 30, 2018. www.multiplesklerose.ch/de/spenden-helfen/benefizevents/welt-ms-tag/
- Dosanto G, et al: The Swiss Multiple Sclerosis Cohort-Study (SMSC): A Prospective Swiss Wide Investigation of Key Phases in Disease Evolution and New Treatment Options. PLoS One 2016; 11(3): e0152347.
- Swiss Multiple Sclerosis Cohort Study (SMSC). 2015. https://clinicaltrials.gov/ct2/show/NCT02433028
- Swiss Multiple Sclerosis Society: Swiss MS Registry: Behavior change after diagnosis. Monthly chart as of March 23, 2017. www.multiplesklerose.ch/de/aktuelles/detail/schweizer-ms-register-verhaltensaenderung-nach-der-diagnose/
- Science et Cité: Switzerland researches. 2018. www.schweiz-forscht.ch
- Competence Center Citizen Science. 2018. www.cc-cs.uzh.ch/de.html
InFo NEUROLOGY & PSYCHIATRY 2018; 16(4): 3-5.











