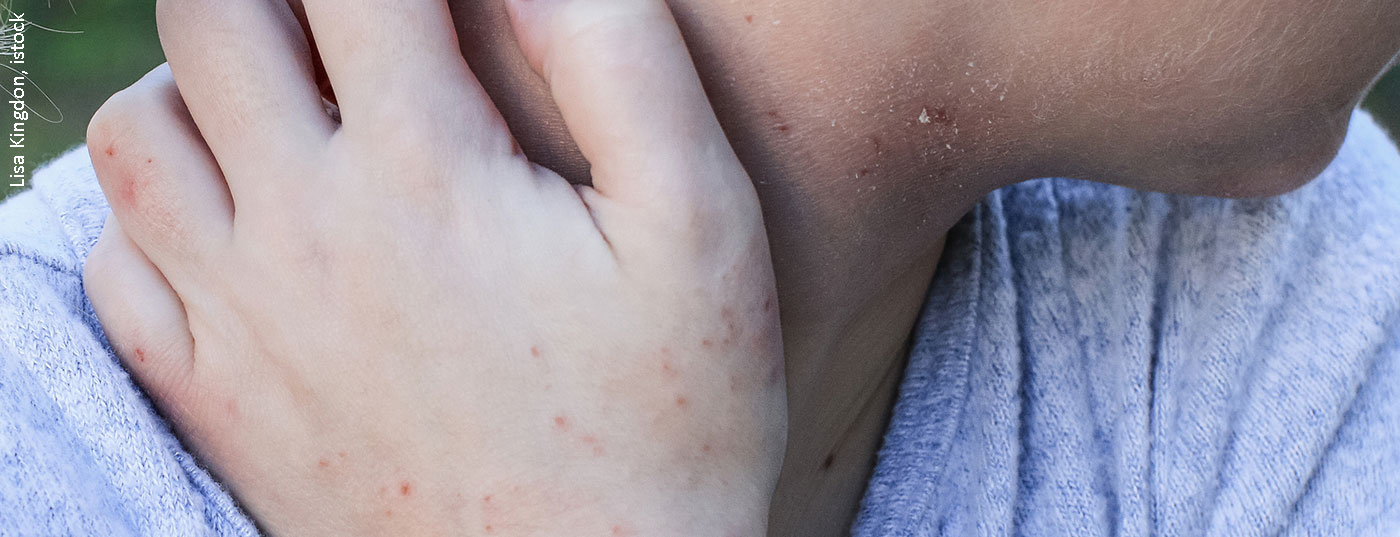
Until now, systemic treatment of atopic dermatitis has been overshadowed by side effect risks. Dupilumab, a biologic that has proven to be highly effective and safe, has been available as an alternative for adults with moderate to severe atopic dermatitis since last year. The market approval of this monoclonal antibody has significantly changed the therapeutic spectrum for this indication.
In contrast to conventional systemic therapy options, biologics very specifically influence certain cellular processes, thereby reducing the risk of side effects. Dupilumab is the first biologic that effectively modifies the pathophysiology of Th2-mediated allergic diseases and has a good safety profile [1]. Until the market approval of this monoclonal antibody, systemic therapies for moderate to severe atopic dermatitis were limited to oral glucocorticoids, ciclosporin, and off-label substances (e.g., azathioprine, mycophenolate mofetil, methotrexate) [2]. Oral glucocorticoids show a marked effect, but longer-term treatment is not recommended due to risks of side effects (e.g., skin and muscle atrophy, osteoporosis, increase in blood glucose levels). Ciclosporin dampens the immune response and thus also inhibits the inflammatory process. This active ingredient can also lead to undesirable side effects when taken over a longer period of time (e.g., impairment of kidney function). Off-label therapeutics (e.g., azathioprine, mycophenolate mofetil, methotrexate) are used in adults in some cases when other treatment is not effective due to inadequate response or side effects.
Th2 cell-mediated processes as a therapeutic target of dupilumab.
By intervening in the disease process in a very targeted manner, the risk of side effects is reduced. Since biologics cannot be absorbed by the mucosa of the gastrointestinal tract due to their structure, they are not taken in tablet form but injected subcutaneously. Dupilumab (Dupixent®) [3] is available as a pre-filled syringe in the prescribed dosage and can be self-administered by patients after medical instruction. The compound, produced by recombinant DNA technology from Chinese hamster ovary cells, is a human IgG4 monoclonal antibody that binds to the alpha subunit of the IL4 receptor, inhibiting IL4/IL13 signaling pathways [4]. This downregulation of Th2-mediated inflammatory processes makes dupilumab effective in various allergic diseases [5]. Type 2 helper (Th2)-cell mediated immune responses play an important role in the pathogenesis of atopic dermatitis by secreting excessive amounts of IL4 and IL13. These two cytokines stimulate the formation of IgE antibodies, resulting in an inflammatory defense response to the irritants. As a result, a vicious circle of disturbed skin barrier, penetration of irritants, inflammation, unbearable itching and scratching and further damage to the skin can develop. Very severe damage to the skin can lead to chronic inflammation (overview 1) and there may be a secondary reduction in barrier function due to inflammation, which phase-dependently involves an interaction between keratinocytes, dendritic cells, cutaneous mast cells, and T cells with corresponding proinflammatory cytokines [2]. At the molecular level, dupilumab led to a reduction in the signature of over 800 genes involved in atopic dermatitis, including those for Th2 chemokines, T cell proliferation, and dendritic cells [6]. Research into the various cellular targets of dupilumab is ongoing [1].
Severity-adapted treatment
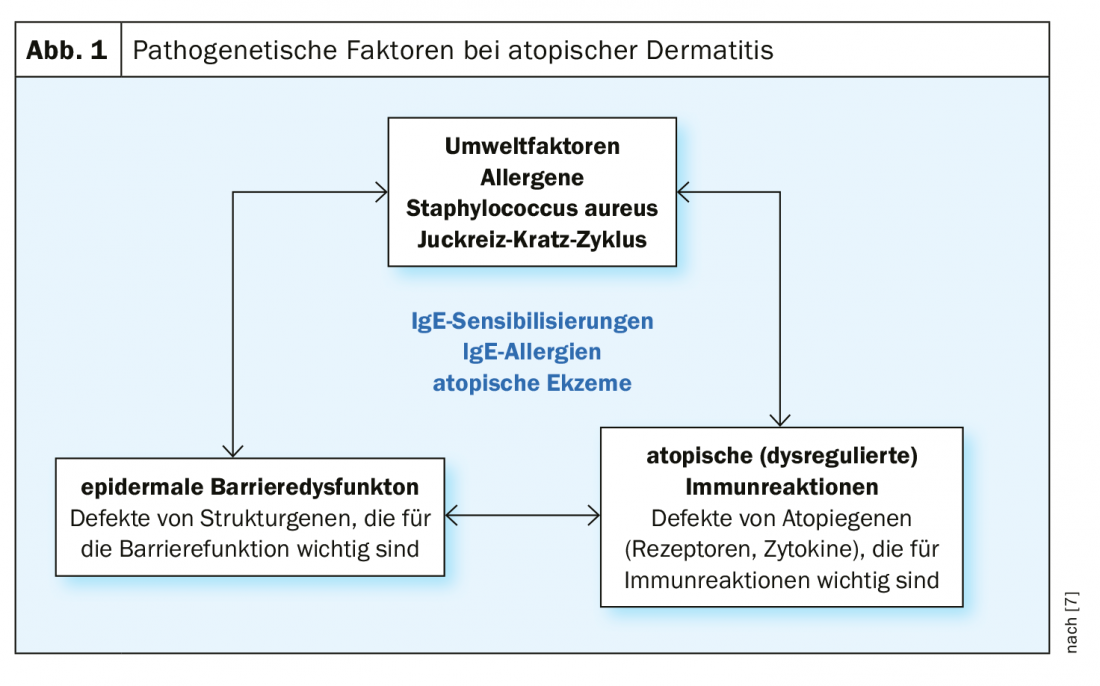
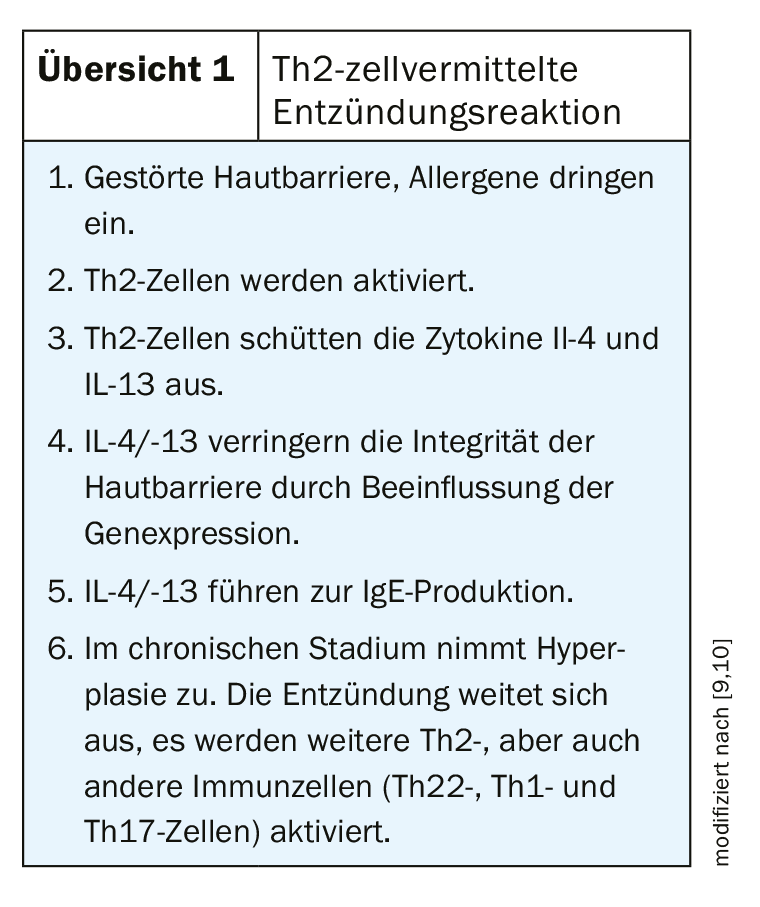
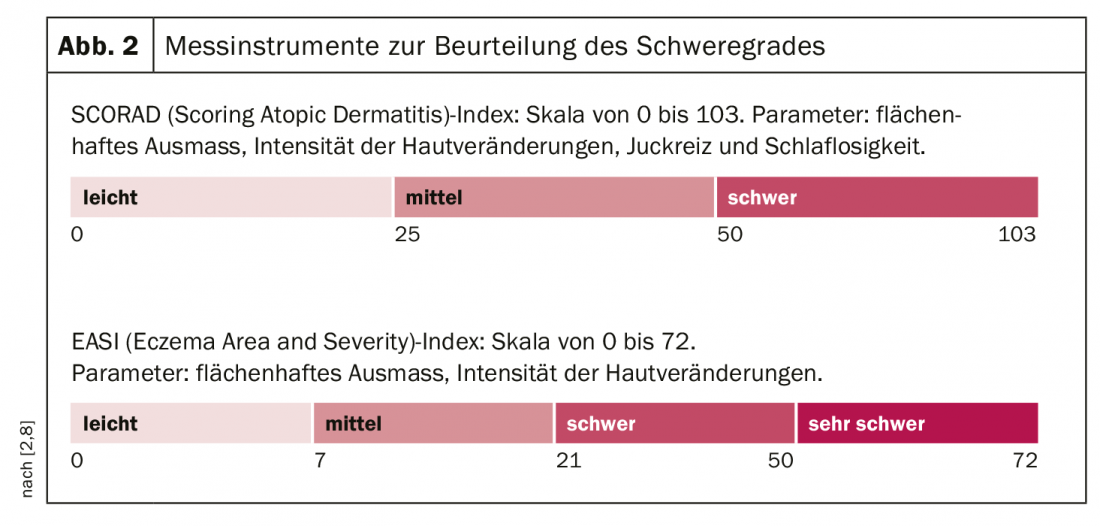
Atopic dermatitis is based on a multifactorial pathogenesis [7]: There are genetic predispositions regarding a disturbance of the epidermal barrier function and of already mentioned complex dysregulations of the immune system with a tendency to develop IgE-mediated sensitizations. (Fig.1). Exogenous trigger factors include irritant substances (e.g., cleaning agents), prolonged or repeated water contact, high air temperature and sweating, and psychosocial stressors [7]. Symptom expression can vary greatly between individuals and in different phases of the disease. About half of all affected individuals experience moderate to severe eczema at least intermittently [8]. Very severe forms of atopic dermatitis affect about 8% of patients. In order to adapt the therapy to the course of the disease in the best possible way, an assessment of the symptomatology including objective and subjective information is necessary. The validated measuring instruments SCORAD (Scoring Atopic Dermatitis) and EASI (Eczema Area and Severity Index) are suitable for assessing the severity and for measuring progression in clinical practice [2]. (Fig. 2). Often unbearable and persistent itching is considered particularly distressing by most patients with severe atopic dermatitis and can lead to a pruritus-scratch cycle. To capture the different dimensions of impaired quality of life, the Dermatologic Quality of Life Index (DLQI) can be used. Especially in severe forms of atopic dermatitis, topical therapy alone is often insufficient, so that systemic treatment is required. The prescription of orally or subcutaneously administered systemic agents is placed on level 4 in the still valid level scheme [2]:
- Stage 1: Dry skin: topical basic therapy/skin care; avoidance or reduction of provoking factors.
- Stage 2: Mild eczema: required measures of stage 1 plus antipruritic and anti-inflammatory agents; mild topical glucocorticoids and/or (from 3 years of age) topical calcineurin inhibitors.
- Stage 3: Moderate eczema: required measures of stages 1-2 plus topical glucocorticoids (class 2 to 3) and/or (from 3 years of age) topical calcineurin inhibitors.
- Stage 4: Persistent severe eczema: required measures of stages 1-3 plus systemic immunomodulatory therapy (tablets/injections to regulate the immune system).
Literature:
- Harb H, Chatila TA: Mechanisms of Dupilumab. Clinical & Experimental Allergy 2020; 50(1): 5-14. https://doi.org/10.1111/cea.13491
- AWMF: Guideline Neurodermatitis [atopic eczema; atopic dermatitis] Development stage: S2k. AWMF Register Number: 013-027 Long version, www.awmf.org
- Swiss Drug Compendium: Dupixent, www.compendium.ch
- European Medicines Agency: Dupixent. Product information, summary of product characteristics, www.ema.europa.eu
- Del Rosso JQ: Monoclonal Antibody Therapies for atopic dermatitis: where are we now in the spectrum of disease management? J Clin Aesthet Dermatol. 2019;12(2): 39-41.
- Hamilton JD, et al: Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2014; 134(6): 1293-1300.
- Trautmann A, et al: Atopic eczema. Allergology in Clinic and Practice 2018, DOI: 10.1055/b-0037-147082, www.thieme-connect.de
- German Skin and Allergy Aid Association, www.dha-schwere-neurodermitis.de
- Gandhi NA, et al: Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2015; 15: 35-50.
- Noda, et al: The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol 2015; 135: 324-336.
DERMATOLOGY PRACTICE 2020; 30(2): 28-29