In 1958, Professor Åke Senning, who was appointed to Zurich a few years later, initiated an unprecedented development in medicine at Karolinska Hospital in Stockholm: the implantation of the first pacemaker in a human being. Over the years, the technology has been further developed and perfected in many ways. After it was thought at the turn of the millennium that hardly anything new could be added now, the last 16 years have shown that this is not the case. Recent developments include cardiac resynchronization therapy (CRT pacemakers), home monitoring, MRI-compatible systems, and the continuing reduction in pacemaker size.
On October 8, 1958, a pacemaker was implanted in a human being for the first time in the world by Professor Åke Senning (1915-2000) at Karolinska Hospital in Stockholm. It was built by the Swedish engineer and physician Dr. Rune Elmqvist (1906-1996). The pacemaker was originally built for an animal experiment. However, the wife of the first patient, Arne Larsson (1918-2001), had pushed with the implanter until the pacemaker was finally implanted because of total AV block with up to 40 syncopes daily (Fig. 1). After implantation, the patient was able to lead a completely normal life without syncope. This was the first time that the consequences of total AV block were shown to be treatable with the aid of a pacemaker [1].

Åke Senning was appointed professor in Zurich and on April 16, 1961, he took over as head of the newly created Surgical Clinic A of the then Cantonal Hospital in Zurich. He brought the technique of pacemaker implantation with him from Sweden. He implanted four pacemakers in Zurich as early as 1961 and six pacemakers in 1962.
The implantable cardiac pacemakers have been further developed. Initially, there were only asynchronous pacemakers (V00), which typically delivered 60 to 70 pulses per minute, regardless of cardiac activity.
In 1968 we were able to implant the first so-called demand pacemakers (VVI/VVT). They had the advantage that they did not induce parasystolia when the patient was self-rhythmic. The VVI pacemaker delivered pulses only when the heart rate was lower than the pacing rate. In order not to infringe the patent of VVI stimulation, Elema has introduced VVT stimulation. This pacemaker stimulated the heart as soon as the heart rate was lower than the pacing rate. At higher heart rates, he delivered QRS-synchronous stimuli that fell within the absolute refractory period of the QRS complex and therefore remained ineffective.
The first atrial synchronous pacemaker (VAT) was also described in 1963 [2]. However, this could only be used after the development of reliable atrial electrodes.
The electrodes
The first pacemakers stimulated the heart via epicardially applied electrodes. Lagergren and Johansson [3] described the first pacemaker implantations with transvenous endocardial leads. They were soon offered not only by Elema, but also by Cordis and Medtronic. The latter manufactured both unipolar and bipolar electrodes. These were rather thick and unwieldy and had different connector systems, so interchangeability of the pacemakers was only possible with adapters. The complication and dislocation rates were also still very high with these electrodes.
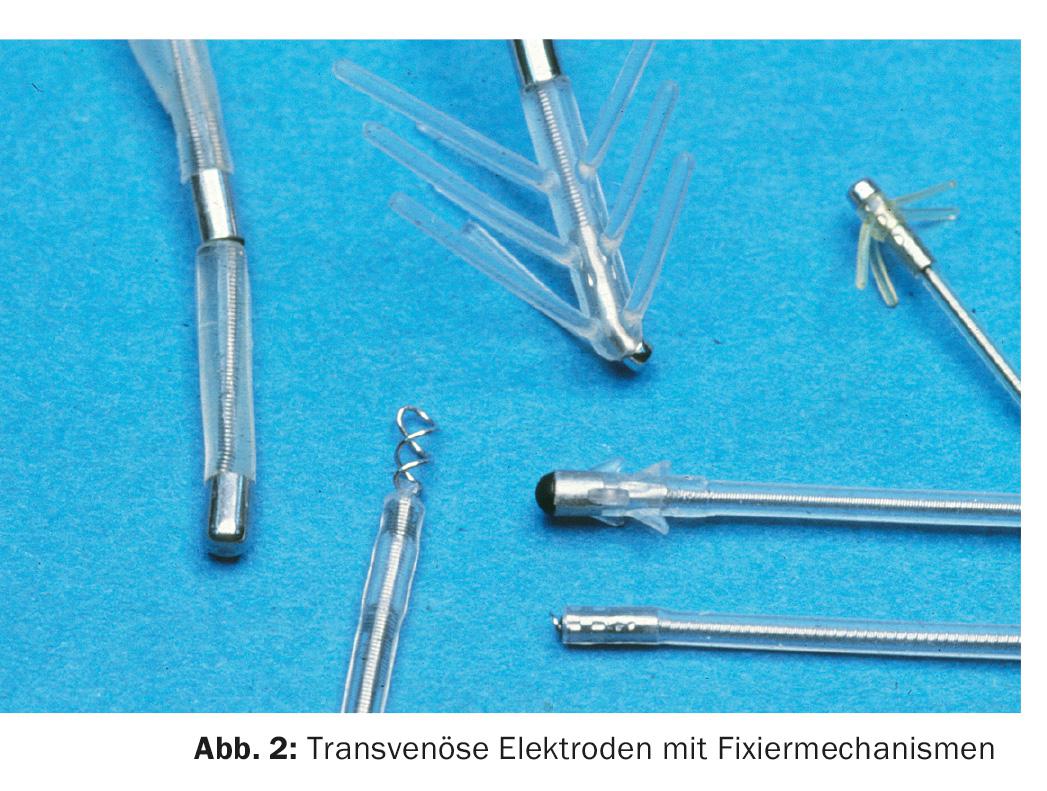
Soon, barbed electrodes or screw mechanisms were added to reduce the high rate of dislocation (Fig. 2) . Lead wires became thinner and more flexible, and controllability during implantation also improved – as did epicardial systems; Medtronic in particular led the way (Fig. 3).
The epicardial leads were popular because the implantation time was predictable, plus they could not dislocate. In 1976, the dislocation rate was around 10%. In Switzerland, 30% of ventricular leads were implanted epicardially. The approach to this was subxyphoidal and the pacemaker was placed abdominally.

The energy source
One of the biggest technical problems in the development of pacemakers was the reliable supply of energy for as long a period as possible. The first pacemaker developed by Senning and Elmqvist in 1958 used two rechargeable nickel-cadmium batteries that had to be recharged through the skin every one to two weeks via an induction coil (placed over the pacemaker).
Apart from the first pacemaker, from 1959 to 1974 the so-called “mercury batteries” of the Mallory company were used almost exclusively as a power source. Since their cell voltage was only 1.35 volts, four to seven cells each with a nominal capacity of 0.95 Ah were connected in series to achieve the output voltage required for stimulation. Due to their relatively large self-discharge in the order of 8-10% per year, the operating life of the pacemakers was only about two to four years. Since hydrogen was produced during the discharge of the cells, neither the battery nor the pacemaker could be hermetically encapsulated without special measures. The battery and electronics were usually encapsulated in epoxy resin, through which the hydrogen could diffuse to the outside.
Pacesetter attempted to use improved rechargeable nickel-cadmium batteries in pacemakers in 1972. The first pacemakers with nickel-cadmium batteries that could be inductively charged by the body required a charging time of four hours per month. Their theoretical service life was up to 20 years. The newly developed bidirectional telemetry system for monitoring battery charge also led to improved service life. Nevertheless, not as many pacemakers were implanted as expected because a newly marketed lithium iodide-based primary battery also achieved up to 15 years of operation without the inconvenience of regular recharging.
This WGL-702 lithium iodide battery was manufactured by Wilson Greatbach for pacemaker use and was first used in a CPI Maxilith 301 pacemaker model in 1973. The cell voltage of the battery was 2.8 volts; thus, only one battery was needed for the pacemaker. The four to five volts output voltage required for stimulation could be generated with the aid of voltage doublers. The self-discharge of the battery was about 1% per year. The battery did not produce any gases during discharge, so the battery and electronics could be hermetically encapsulated in a metal housing.
As a competitor to the lithium iodide battery, the lithium/thionyl chloride battery was developed and first used in pacemakers by the Arco company in 1974. The cell voltage was 3.7 volts. The high energy density of the cell allowed relatively small mechanical dimensions. However, the fact that the battery voltage quickly collapsed at the end of the operating time soon turned out to be a disadvantage for pacemaker operation, which is why a necessary pacemaker replacement could not be foreseen in time. As a result, a large number of pacemakers worldwide had to be replaced prematurely. In Switzerland, too, twice as many pacemaker changes were performed in 1982 as in the previous year.
Developments in the 70s
The first pacemaker with “programmable” frequency (type 5842) was manufactured by Medtronic-Chardac in 1969. However, the “programming” of the frequency was somewhat more cumbersome than today. A sterile three-pronged needle was inserted under local anesthesia through the patient’s skin through a silicone nipple on the pacemaker to the frequency-determining potentiometer. By turning the needle, the potentiometer and thus the pacing frequency could be changed. In the same way, the intensity of the stimulus amplitude was also changed in a later Medtronic model. A few years later, an improvement was made by magnetically transmitting the rotary motion to a mechanical reduction gear in the pacemaker, via which the potentiometer was rotated.
This was soon followed by programming using magnetic pulses. In 1973, the first programmable cardiac pacemaker (VVIP) with two CMOS integrated circuits was developed by the Cordis company. The stimulation frequency as well as the stimulus intensity (amplitude or pulse duration) could be changed in six steps each with magnetic pulses via a magnetic switch (reed switch).
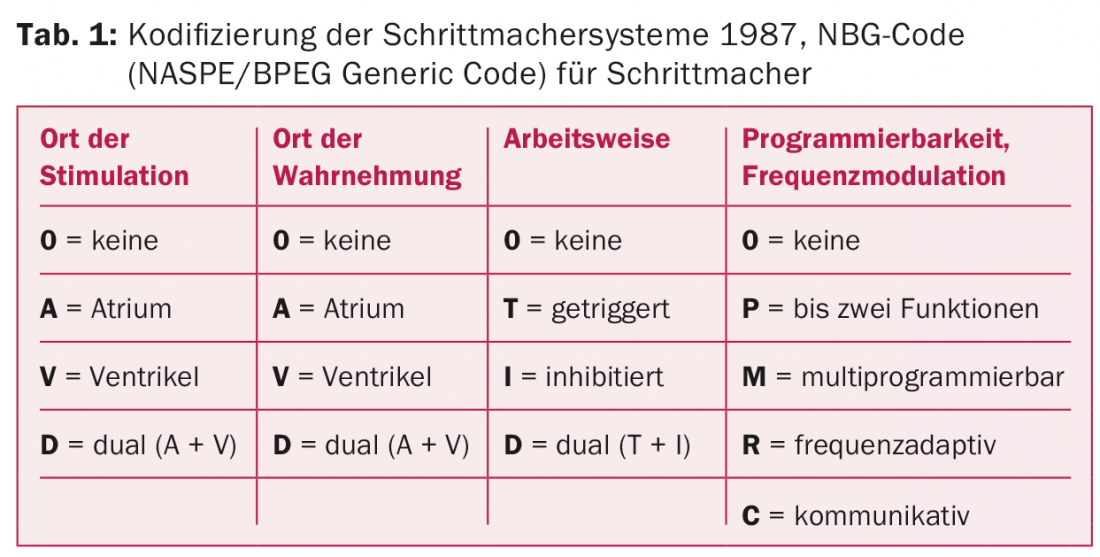
The growing complexity of the systems soon required a simple description of the types of functions. This was initially done in 1974 with the ICHD (Inter-Society For Heart Disease Resources) code consisting of three letters. Because of the development of programmable pacemakers, this code had to be expanded to a five-letter code in 1981. In 1987 and 2000, the codification was revised and the resulting NBG code (NASPE/BPEG Generic Code) is still valid today (Tab. 1).
In 1978, the first multiprogrammable pacemakers AAIM and VVIM were developed. Here, virtually all pacing parameters could be set as needed. Unfortunately, the programming of the parameters was measured only indirectly. The confirmation was missing.
Pacemaker implantations in Switzerland
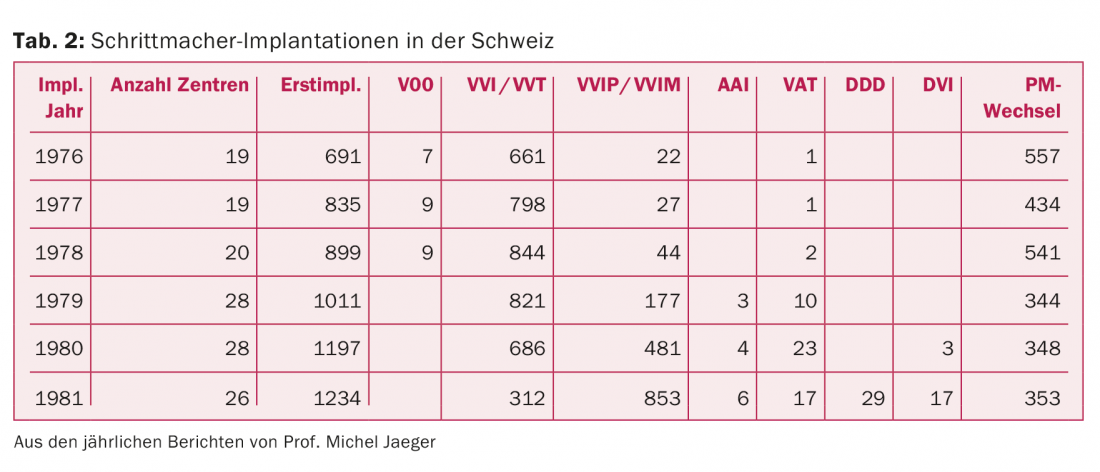
In 1976, 19 hospitals in Switzerland had already implanted pacemakers (tab. 2) . At that time, pacemaker implantations were still associated with numerous technical and surgical problems. Therefore, in 1975, the Swiss Society of Cardiology asked Professor Michel Jaeger from Lausanne to take over the coordination as delegate for pacemaker problems and to statistically record the implantation figures as well as the problems on a nationwide annual basis. He held this office until 1981. In 1980, the pacemaker working group of the SGK was founded and Prof. Jaeger served as its president from 1980 to 1983 (see www.pacemaker.ch).
In 1978, Luc Tissot, of Tissot watches, founded Precimed S.A. in Le Locle together with Branco Weiss, founder of Kontron (a subsidiary of Hoffmann-La Roche). The first industrially manufactured pacemakers in Switzerland, named Precilith, were built at the Tissot watch factory. All pacemakers demonstrated high reliability and long service life. When the development of the first programmable pacemakers required a major investment, the business was sold to the American pacemaker company Intermedics in 1983.
By the end of the 1970s, the number of companies offering their products in Switzerland had risen to twelve.
The first pacemaker with bidirectional telemetry
In 1979, the first pacemaker with bidirectional telemetry (Programalith) was developed by the Pacesetter company. Pacesetter benefited from the experience they had gained with rechargeable batteries. The pacing parameters could be changed with the programmer by electromagnetic signals, and the programming was confirmed telemetrically. In addition, measurement data such as battery voltage or electrode resistance were transmitted from the pacemaker to the programmer, allowing better monitoring of battery condition and electrodes.
The physiological stimulation
In the 1980s, physiological stimulation of the heart came more and more to the fore. It was discovered that sequential or atrial pacing achieved better hemodynamics than VVI pacing.
For the time being, AAI, VAT, and DVI pacemakers were implanted. The first DDD pacemaker was developed by Medtronic in 1980. This was still quite large, non-programmable and weighed 148 g. The first multi-programmable dual-chamber pacemaker was introduced in 1981 by Biotronik (Diplos-03) and implanted in Switzerland.
In 1984, Medtronic developed the first Activitrax frequency-adaptive pacemaker with a built-in sensor that accelerated the pacing rate when the patient was physically active. The vibrations generated when the patient moved were received by a piezoelectric crystal, and the pacing frequency was changed according to the intensity of the signals. Soon, other manufacturers entered the market with a wide variety of activity sensors. An approximate physio-logical response was achieved with the Meta MV respiratory minute volume control from Telectronics.
Initially, only single-chamber systems were controlled with the sensor, but later the rate-responsive mode was also used with dual-chamber systems.
By the end of the 1980s, virtually all types of pacemakers known today had been manufactured and implanted (Table 3).

In 1985, the industry finally agreed on a uniform VS-1 (Voluntary Standard) connector system, which later led to the IS-1 connector (3.2 mm instead of 5 or 6 mm in diameter). This not only simplified the pacemaker change, but also significantly reduced the size of the pacemakers.
Developments in the 1990s
In the 1990s, programming devices in particular were further developed and optimized. Bidirectional telemetry has been added by virtually every manufacturer, and patient monitoring has become increasingly easy. The data could be printed with the printer built into the programmer. From the telemetrically transmitted values, the programmer calculated the expected operating time of the pacemaker and the remaining battery capacity. The intracardiac potentials could be visualized and their magnitude could be measured. The stimulus threshold of the connected electrodes could also be determined manually or fully automatically. Stimulus intensity was automatically adjusted to the stimulus threshold, extending the operating time of the pacemaker – possibilities that were not available years before. Patient safety has thus been improved more and more.
The pacemakers not only became “smarter,” they became smaller and smaller with the same or longer life span. While the first DDD pacemaker weighed 150 g in 1980, a DDDR pacemaker with bidirectional telemetry from the same company weighed only 32 g in 1997. This has made not only patient monitoring but also pacemaker implantation increasingly easy.
Developments after the turn of the millennium
Due to mergers and company mergers, the number of companies offering their products in Switzerland has decreased to five.
It was thought that everything was developed and nothing new could be added. But the development continued and more and more automatisms and algorithms were added to both the pacemakers and the programmers. Diagnostic functions support follow-up control and optimal adaptation of the pacemaker function to the patient’s needs.
Below are some examples of the development in recent years.
Cardiac resynchronization therapy (CRT pacemaker)
For cardiac resynchronization, trials with ordinary dual-chamber pacemakers were already undertaken in the 1990s. Using a Y-adapter and epicardial electrodes, both ventricles were stimulated. Later, the epicardial electrode was replaced by the coronary sinus electrode. The disadvantage of this system was that you could not set the AV time left and right independently.
This capability was built into the first InSynk three-chamber pacemaker, launched by Medtronic in 2001.
With these pacemakers, two ventricular leads could be connected, and AV times could be programmed independently. The left ventricular lead was implanted venously via the coronary venous sinus at the level of the lateral wall of the left ventricle. Premature stimulation of the delayed-excited posterolateral wall restored resynchronization of the impaired ventricular contraction.
Today, biventricular systems are offered by all companies. In 2015, approximately 4% of first implantations in Switzerland were CRT pacemakers.
Home Monitoring
Home monitoring introduced by Biotronik can minimize the number of visits to the doctor by a patient with an implanted pacemaker. Depending on the setting, all the patient’s health-related information is transmitted to the practice or clinic once a day at a specific time and stored for regular progress monitoring. They thus allow the physician to monitor the patient’s condition fully automatically and enable early detection of serious complications. Events such as arrhythmias and system abnormalities are reported directly to the physician rather than being uncovered during the next routine exam. This allows the doctor to react immediately and contact the patient if necessary.
MRI compatible systems
During MRI examination of a patient with an implanted pacemaker or ICD, high voltages can be generated in the electrodes by induction, causing burns at the electrode tip on the myocardium. The device may also be damaged or its function disrupted for the same reason.
Newly developed pacemaker systems and electrodes allow MRI examination of the patient. Special protective mechanisms and electrode configurations ensure patient and device safety.
However, MRI compatibility should be handled with caution. The entire system, i.e. both the pacemaker or ICD and the connected electrodes, must be MRI-compatible. There must also be no idle non-MRI resistant electrode not connected to the device. It is best to contact the clinic that implanted the pacemaker prior to an MRI exam.The manufacturer can also provide competent information about MRI compatibility.
Smaller and smaller pacemakers
Since June 2015, Medtronic’s latest development, the Micra electrodeless intracardiac VVIR pacemaker, has also been approved and available in Switzerland. It is placed in the right ventricle using a catheter and anchors to the myocardium using Nitinol hooks. Electronics and battery are housed in the pacemaker. It is cylindrical, 26 mm long, 6,7 mm wide in diameter and has a weight of 1,8 g. It is fully programmable, a frequency histogram is stored, and it has all the same features as a pacemaker with transvenous electrodes. In 2015, 44 such systems were already implanted in Switzerland.
Closing words
In this brief summary, an attempt has been made to show the individual development stages and hurdles of the past 58 years, without claiming to be exhaustive. Where the further development will lead, we cannot foresee, at most we can guess.
Literature:
- Senning A: Discussion of a paper by Stephenson SE, et al: Physiologic P-Wave Cardiac Stimulator. J Thorac Cardiovasc Surg 1959; 38: 604-609. Read at the thirty-ninth Annual Meeting of The American Association of Thoracic Surgery, April 21-23, 1959, Los Angeles.
- Nathan DA, et al: An implantable synchronous pacemaker for the long-term correction of complete heart block. Circulation 1963; 27: 682-685.
- Lagergren H, et al: One hundred cases of treatment for Adams-Stokes syndrome with permanent intravenous pacemaker. J Thorac Cardiovasc Surg 1965; 50(5): 710-714.
CARDIOVASC 2016; 15(3): 4-9