For the majority of patients with respiratory diseases, inhalation is the most effective treatment method. Compared to systemic routes of therapy (oral, i.v., i.m., s.c.), it offers a distinct treatment advantage by delivering the active agent directly to the lungs. However, a prerequisite for this is that both practitioner and patient know how to use the inhaler correctly.
With inhalation, smaller doses can be administered compared to other methods. The onset of action occurs rapidly and the incidence of side effects is low, according to other advantages listed by Prof. Dr. Omar S. Usmani of Imperial College London at the European Respiratory Congress (ERS) [1]. So the primary question is how to get the right dose to the right place: “You can have the best drug, but if the inhaler device doesn’t deliver the drug effectively and accurately to the lungs, then the drug has no efficiency!” To achieve this goal, three aspects are eminent, he said:
- Formulation and chemistry of the aerosol
- Development and design of the device
- Training and application technique of the patient.
Formulation
The size, shape and density of the particles to be inhaled are of importance. Low-density porous particles are used in inhaled antibiotics and inhaled insulin, and needle-shaped particles based on asbestos and filament viruses are being developed for use in inhaled corticosteroids.
Particle size affects the total amount of drug that enters the lungs and exactly where it is delivered. Most devices used in practice achieve a respirable range of 1 µm-5 µm [2]. As early as 2005, a group led by Prof. Usmani tested three different particle sizes (1.5 µm, 3.0 µm and 6.0 µm) on asthma patients. It was found that the application of smaller particles achieved better lung deposition and greater penetration of these aerosols into the lungs (Fig. 1) [3]. The same result was later shown in patients with COPD: smaller particles achieve better lung deposition overall and better distribution through the airways.
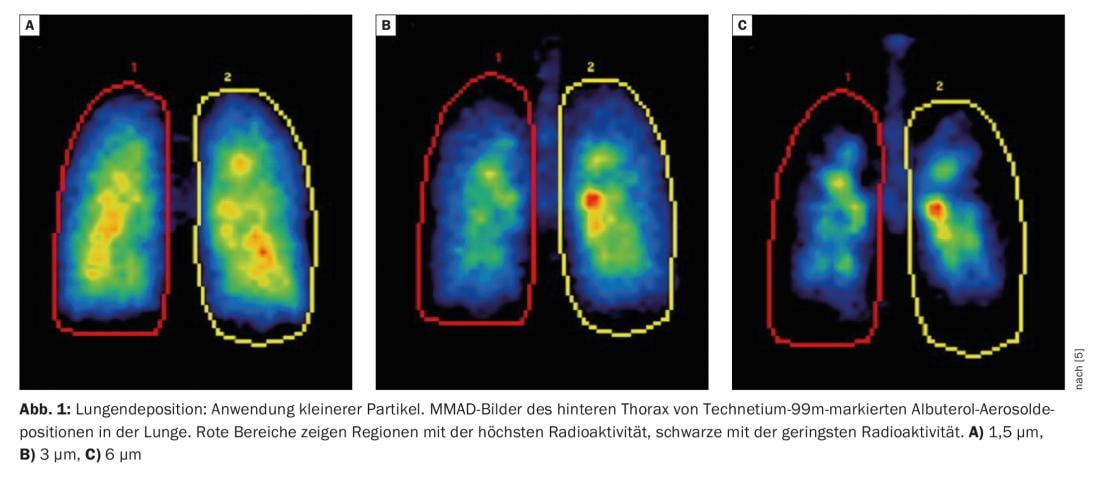
Prof. Usmani pointed out that the 2022 GOLD report [4] also notes the importance of peripheral deposition and extra-fine treatment, “For drug delivery to the lower airways and lungs, particle size (mass median aerodynamic diameter) can be fine (2-5 µm) or extra fine (<2 µm), which means the total respirable fraction (particles <5 µm) and the amount and location of drug deposition is affected (more peripheral deposition with extra-fine particles).”
In the last three to four years, there has been great progress in the clinical development of inhaled nanoparticle therapeutics, the expert said. The concept behind this consists of a protective layer, the therapeutic part and targeting molecules, i.e. molecules on the surface of the nanoparticles that guide the overall package to its target. Such nanotherapeutics are under development for inhaled corticosteroids.
Device development and technology
Inhaler devices in use today are mostly pressurized metered dose inhalers (pMDI), dry powder inhalers (DPI), nebulizers, and soft mist inhalers (SMI). Innovations that physicians and patients can look forward to include.
- MDI, which are soft mist (since the emission of the fine mist allows to transport the aerosol better and more accurately into the lungs),
- antistatic spacers that do not require preparation and can be applied immediately by the patient,
- Dry powder nebulizers that are battery operated, very user friendly, have a dose reminder and provide feedback,
- reusable soft mist inhaler,
- smaller nebulizers that can be held in the hand.
Prof. Usmani particularly highlighted “smart nebulizers,” whose mode of operation is already known from the treatment of pulmonary hypertension and cystic fibrosis: portable, battery-powered, vibrating mesh nebulizers that instruct users to inhale deeply and slowly, constantly taking into account changes in patients’ breathing. The devices can emit the aerosols whenever the user actually inhales, achieving a deposition of 50-70%. The loss of aerosol during exhalation is thus minimized.
Training and application technology
Suboptimal use of the inhaler affects clinical efficiency. This sounds like a matter of course, but it still does not seem to have fully arrived in practice, as Prof. Usmani lamented. This refers not only to the often lacking compliance of patients, but also to the technical handling and use of the inhaler.
For pMDI devices, the most common mistakes are not inhaling slowly enough, inhaling deeply enough, or coordinating the two. Most patients would inhale too quickly and thus would not be able to achieve the best possible effect. Dry powder inhalers, on the other hand, would not inhale forcefully and deeply enough. DPIs would require a flow of at least 60 l/min to be adequate. In real life, he said, this is not achievable by many patients.
Prof. Usmani presented the PIFotal study, which investigated peak inspiratory flow (PIF) in COPD patients (n=1434) using dry powder inhalers for maintenance therapy [5]. Optimal PIF versus suboptimal (sPIF) were contrasted. sPIF was defined as a typical PIF lower than required for the device. Inhalation technique was assessed and graded by standardized evaluation of video recordings.
Patients were further categorized into three clinically relevant subgroups based on their PIF:
- “Able and willing”: patients with optimal PIF
- “Can, but don’t”: patients with a typical PIF below the optimal PIF for their device, but who are able to perform a maximum PIF that is equal to or even higher than in the optimal PIF group.
- “Not possible”: patients who have both their typical and maximum PIF below the optimal PIF for their device.
71% of patients had an optimal PIF, and 29% of all participants did not generate an optimal PIF for their DPI during a typical inhalation procedure [6]. 16% of these showed that they were actually able to generate an optimal PIF for their device but did not achieve it during the inhalation process. This may indicate that sPIF is a potentially treatable feature in COPD management. The remaining 13% fell into the last group (“Not possible”), who were not able to achieve the optimal PIF for their Device even with their maximum PIF. This issue could likely be solved in the future with multi-dose electronic DPIs, the pulmonologist said. Such dry powder inhalers could use integrated digital sensors to assess the inhalation while the patient is still performing it and take into account parameters such as PIF to provide appropriate feedback to the user.
Finally, Prof. Usmani also admonished his colleagues: Doctors often have no knowledge about inhalers, because it is not taught at universities and colleges. Accordingly, many do not know which device is suitable for which type of patient. Of more than 6000 pulmonologists and allergists, primary care physicians, respiratory therapists, nurses, and pharmacists surveyed, only 12% knew the proper application techniques or how to choose the appropriate devices. In addition to further technical development and patient training, physician self-reflection is also a factor on the path to optimal inhaler use.
Congress: ERS Congress 2022
Literature:
- Symposium: The future of inhalation therapy in COPD. European Respiratory Society (ERS) International Congress 2022, Barcelona, Sept. 4, 2022.
- Chrystyn H: Anatomy and physiology in delivery: can we define our targets? Allergy 1999; 54: 82-87; doi: 10.1111/j.1398-9995.1999.tb04393.x.
- Usmani OS, Biddiscombe MF, Barnes PF: Regional lung deposition and bronchodilator response as a function of β2-agonist particle size. Am J Respir Crit Care Med 2005; 172: 1497-1504; doi: 10.1164/rccm.200410-1414OC.
- GOLD Report 2022, p. 58; https://goldcopd.org/2022-gold-reports-2; last accessed Nov. 9, 2022.
- Leving M, Wouters H, de la Hoz A, et al: Impact of PIF, inhalation technique and medication adherence on health status and exacerbations in COPD: protocol of a real-world observational study (PIFotal COPD Study). Pulm Ther 2021; 7(2): 591-606; doi: 10.1007/s41030-021-00172-7.
- Kocks JWH, Wouters H, Bosnic-Anticevich S, et al: Factors associated with health status and exacerbations in COPD maintenance therapy with dry powder inhalers. npj Prim Care Respir Med 2022; doi: 10.1038/s41533-022-00282-y.
InFo PNEUMOLOGY & ALLERGOLOGY 2022; 4(4): 22-24 (published 12/1/2012, ahead of print).











