Janus kinase (JAK) inhibitors inhibit signal transduction of numerous proinflammatory cytokines with varying selectivity. With baricitinib, upadacitinib and abrocitinib, three oral JAK inhibitors are currently approved in Switzerland for the treatment of moderate to severe atopic dermatitis.
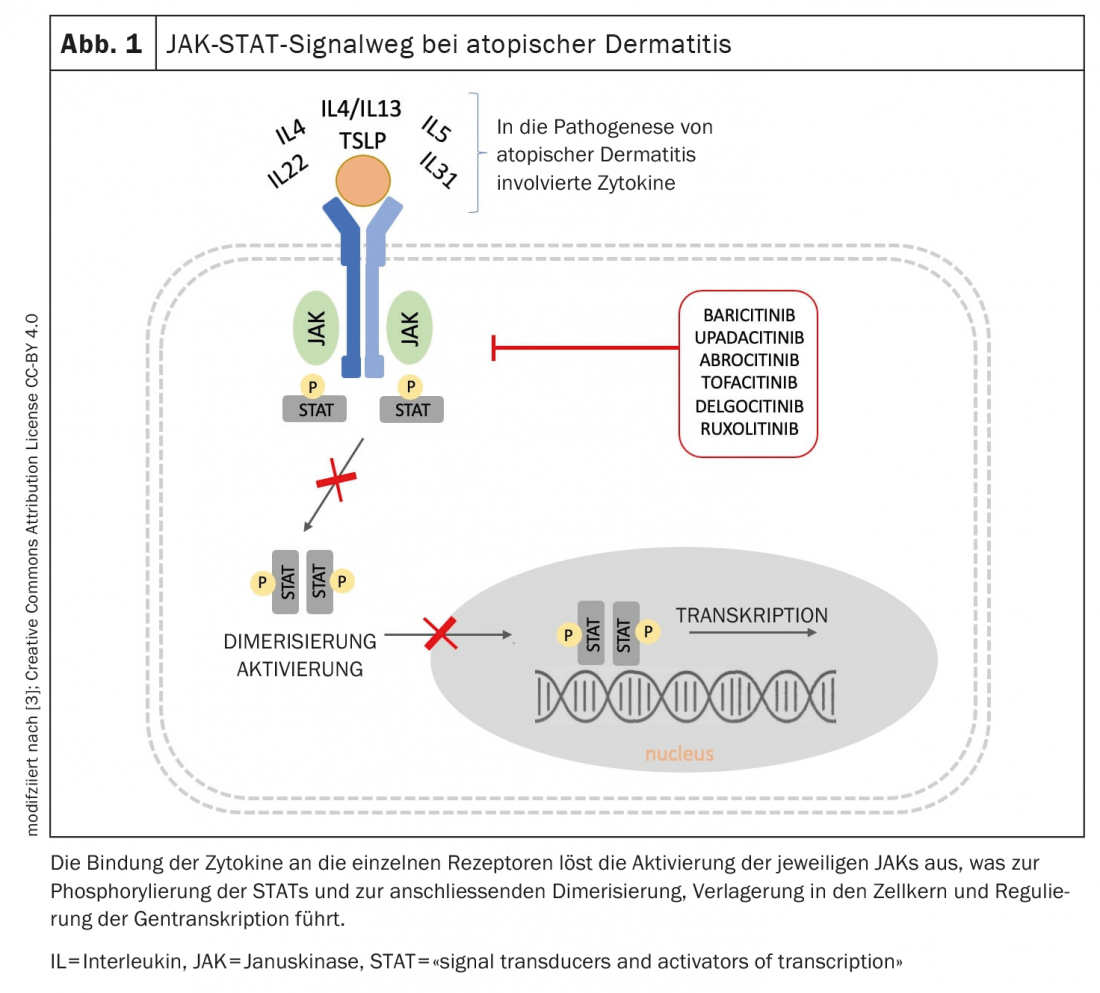
In recent years, an increasingly better understanding of the pathogenesis of atopic dermatitis has led to the development of novel therapeutic molecules targeting key inflammatory components of the disease [3]. Janus kinases (JAKs) are enzymes that transduce intracellular signals from cytokine or growth factor receptor interactions at the cell membrane to control cellular processes of hematopoiesis and immune cell function. Within the intracellular signaling pathway, JAKs phosphorylate and activate signal transducers and activators of transcription (STATs), which in turn activate gene expression within the cell. Signal transduction within the cell occurs via the JAK-STAT signaling pathway (Fig. 1), ultimately leading to the expression of additional proinflammatory cytokines [3]. By inhibiting the enzymatic activity of one or more Janus kinases, phosphorylation and activation of STATs are reduced. The currently approved JAK inhibitors modulate, among others, interleukin(IL)-4 and IL-13, two key cytokines in the pathogenesis of atopic dermatitis.
Rapid onset of itch relief
Janus kinase inhibitors are characterized by a rapid onset of action, as well as rapid and sustained relief of itching. In addition to a reduction in the number of exacerbations, an improvement in quality of life with regard to depression, anxiety and sleep disturbances is one of the therapeutic effects of JAK inhibitors. The oral dosage form as a tablet is easy for patients to handle. In Switzerland, baricitinib (Olumiant®), upadacitinib (Rinvoq®) and abrocitinib (Cibinqo®) are currently approved for the indication area of atopic dermatitis [1].
Baricitinib is a selective and reversible inhibitor of JAK1 and JAK2. In the BREEZE-AD1 and BREEZE-AD2 monotherapy trials, a significantly higher proportion of patients receiving baricitinib 4 mg achieved an IGA 0 or 1, an EASI-75*, or an improvement of ≥4 points on the pruritus NRS at week 16
#
compared with placebo [1]. A significantly higher proportion of patients randomized to baricitinib 4 mg achieved ≥4 points improvement in NRS within the first week of treatment compared with placebo (p<0.001).
* EASI-75 = min. 75 percent improvement in Eczema Area and Severity Index.
# The Peak Pruritus NRS [2] captures itchiness, with a value of 0 corresponding to no itchiness and a value of 10 corresponding to the worst imaginable itchiness. An improvement of ≥4 points is considered a relevant improvement.
Upadacitinib is a is a selective and reversible inhibitor of JAK1. In the MEASURE UP monotherapy trials, a significantly higher proportion of patients treated with upadacitinib 15 mg achieved a vIGA-AD response of 0 or 1 and an EASI-75 at week 16 compared with placebo [1]. During the same period, a significantly higher proportion taking upadacitinib manifested a reduction in pruritus of ≥4 points on the pruritus NRS compared with placebo. Significant differences in itch reduction in favor of the upadacitinib arm were already evident after week 1 (p<0.001).
Abrocitinib is also a specific JAK1 inhibitor. In the phase III MONO-1 and MONO-2 trials, treatment with abrocitinib 100 mg (1× d) achieved both primary endpoints IGA 0 or 1 and/or EASI-75 in a significantly greater proportion at week 12 [1]. In combination with TCS**, the JAK inhibitor proved significantly superior to placebo in the COMPARE trial at week 16 with respect to these two endpoints. A reduction in itch by ≥4 points on the pruritus NRS manifested with abrocitinib versus placebo as early as week 2 in a significantly higher proportion of study participants.
** TCS=topical steroids
Literature:
- Drug Information, www.swissmedicinfo.ch, (last accessed Aug. 18, 2022).
- Yosipovitch G, et al: Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol 2019; 181(4): 761-769.
- Tsiogka A, et al: The JAK/STAT Pathway and Its Selective Inhibition in the Treatment of Atopic Dermatitis: A Systematic Review. J Clin Med 2022; 11(15): 4431.
DERMATOLOGY PRACTICE 2022; 32(4): 28