Narrow complex tachycardia is defined by a heart rate ≥100 bpm and a QRS duration of ≤0.12 sec. The site of origin is usually supraventricular. Narrow complex tachycardia often occurs recurrently and is a common reason for presentation to the emergency department or family practice.
Narrow complex tachycardia is defined by a heart rate ≥100 bpm and a QRS duration of ≤0.12 seconds. The site of origin of narrow complex tachycardia is usually supraventricular, that is, above the His bundle. Narrow complex tachycardia often occurs recurrently and is therefore a frequent reason for presentation to the emergency department or family practice. Figures from the United States estimate the incidence of narrow complex tachycardia at 35 cases/100,000 patients/year (excluding atrial fibrillation, atrial flutter, and multifocal atrial tachycardia, which are not the focus of this article) [1]. The 12-lead ECG and, if necessary, a longer rhythm strip still represent the basis for establishing the diagnosis.

Forms of narrow complex tachycardia
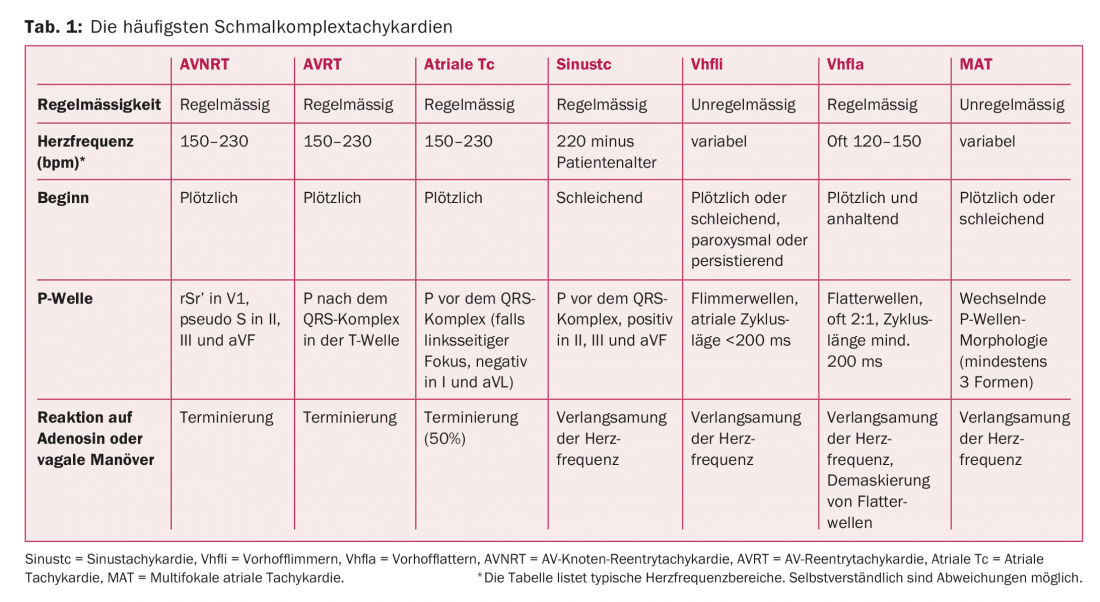
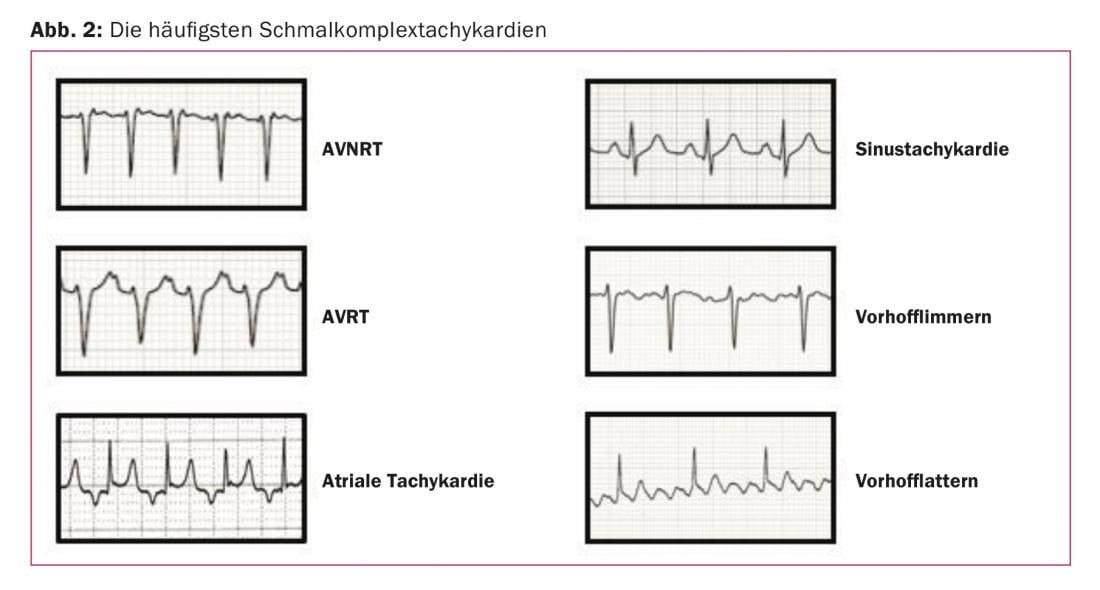
Table 1 and Figure 2 provide an overview of the most common narrow complex tachycardias. Most regular narrow complex tachycardias result from circling excitations (reentry) and are classified based on the location of the reentry circle. In AV nodal reentry tachycardia (AVNRT), the presence of so-called “dual AV nodal conduction” is responsible for the development of a reentry mechanism. This is understood to mean two functionally separate pathways; a slow pathway with a short refractory period (“slow pathway”) and a fast pathway with a long refractory period (“fast pathway”). Triggered by an extrasystole, there is a circular excitation and, in the majority of cases, tachycardia with antegrade conduction via the slow pathway to the ventricles and via the fast pathway retrograde back to the atrium (“slow-fast”). In AVNRT, the most common form of regular supraventricular tachycardia (circa 60% of all cases), the age of first manifestation is usually between 18-40 years [2,3]. Women are more frequently affected.
The second most common cause (30% of cases) is AV reentrant tachycardia (AVRT) [2,3], which has a congenital atrio-ventricular (“accessory”) pathway as a substrate. A distinction is made between the preexcitation or WPW syndrome, where the accessory conduction pathway is evident even in normal sinus rhythm by premature antegrade excitation of the ventricle (delta wave), and the strictly retrograde conduction pathway, in which the ECG is normal in the interval. Although these pathways conduct strictly retrograde, they can maintain a fast AVRT.
Atrial tachycardias account for the remaining 10% of cases and often have a focal origin [2,3]. Less commonly, scar-related intra-atrial reentry mechanisms, e.g., after cardiac surgery, trigger the tachycardia.
Clinic
Anamnestically, AVNRT and AVRT paroxysmal tachycardias can be essentially differentiated by three characteristics:
- They occur suddenly (triggered as by flipping a light switch), last from seconds to several minutes, rarely hours, and usually end abruptly. Heart rates of 150-230 bpm are typically achieved.
- Palpitations are felt in the chest area or more commonly in the neck, in the area of the carotids. A “frog sign” may be seen on clinical examination, if appropriate. This is pulsation of the jugular veins due to atrial contraction against the closed tricuspid valve.
- If the tachycardias persist for a longer period of time, the patient needs to relieve water more frequently (increased ANP release in the atrium due to stretching).
12-lead ECG interpretation strategy
1. regularity: Once narrow complex tachycardia has been identified, the next step should be to assess regularity, which in the majority of cases allows differentiation from atrial fibrillation. Regular tachycardia varies <5% from beat to beat. If known, e.g., during telemetric monitoring in the emergency department or long-term ECG, special attention should be paid to the onset and termination of tachycardia.
2. heart rate: In principle, a causal assignment based on heart rate is not possible. However, it gives important clues in the correct interpretation of the arrhythmia. In sinus tachycardia, the maximum heart rate is basically 220 bpm minus the patient age. In typical atrial flutter, the atrial frequency of the macro-reentry circuit in the right atrium is usually 250-300 bpm. Because tachycardic atrial flutter is often transmitted 2:1 to the ventricles, a ventricular rate of 120-150 bpm is characteristic. Therefore, if ventricular rates are very rapid (e.g., 240-260 bpm), atrial flutter with 1:1 conduction to the ventricles should also be considered. However, not only the ventricular frequency provides important information: The variability of atrial cycle lengths and the morphology of flutter waves provide further clues in the differentiation from atrial fibrillation. If the atrial cycle length is shorter than 200 ms, typical atrial flutter is almost never present, and atrial fibrillation is more likely to be considered.
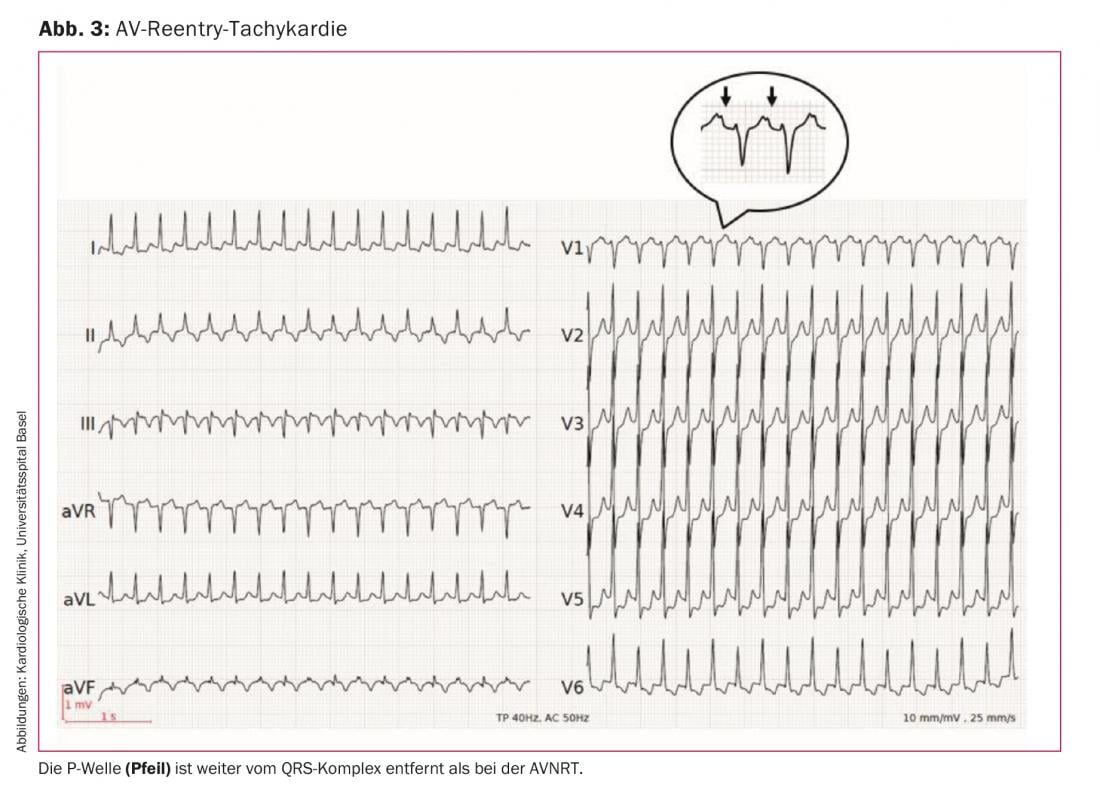
3. atrial excitation: visible P waves preceding the QRS complex are present in sinus tachycardia, atrial tachycardia, and multifocal atrial tachycardia. P waves after the QRS complex are found in AVNRT and AVRT (table 1) . However, the P waves may be hidden in the QRS complex, especially in very fast supraventricular tachycardia. In our example (Fig. 1) , a P wave appears at the end of the QRS complex, best visible in lead V1. This configuration is termed pseudo-rSr’, analogous to incomplete right bundle branch block. A retrograde P wave can also be detected in certain cases in the inferior leads as a pseudo-S. The presence of a P wave at the end of the QRS complex reflects very rapid ventriculo-atrial conduction (VA time) and is thus indicative of AVNRT. Because an accessory pathway only takes a longer path over the entire ventricular activation, such a short VA time is not possible with AVRT. This results in the P wave being farther away from the QRS complex (Fig. 3) than in AVNRT.
4. response to vagal maneuvers or adenosine: the response to vagal maneuvers or adenosine is extremely useful not only therapeutically but also diagnostically, which is why it is mandatory that it be performed under continuous ECG monitoring. The most common vagal maneuvers are the carotid sinus massage (cave carotid stenosis, auscultation mandatory) and the Valsalva maneuver. The principle is simple: the AV node is vagally innervated. Vagal stimulation causes conduction slowing (or AV block), which terminates AV nodal-dependent tachycardia (AVNRT, AVRT) or unmask the underlying atrial rhythm (atrial flutter, atrial tachycardia, sinus tachycardia). It is crucial that the pressure is maintained for at least 15 seconds during the carotid massage. In cases of prolonged tachycardia or high endogenous stress, successful performance of vagal maneuvers may be difficult (success rate circa 50%), so adenosine may be used as an alternative. Adenosine is a very short-acting purine nucleoside that has a negative dromotropic effect on the AV node, usually leading to complete AV block during a few seconds. However, in addition to AVNRT and AVRT, more than 50% of atrial tachycardias are terminated by adenosine.
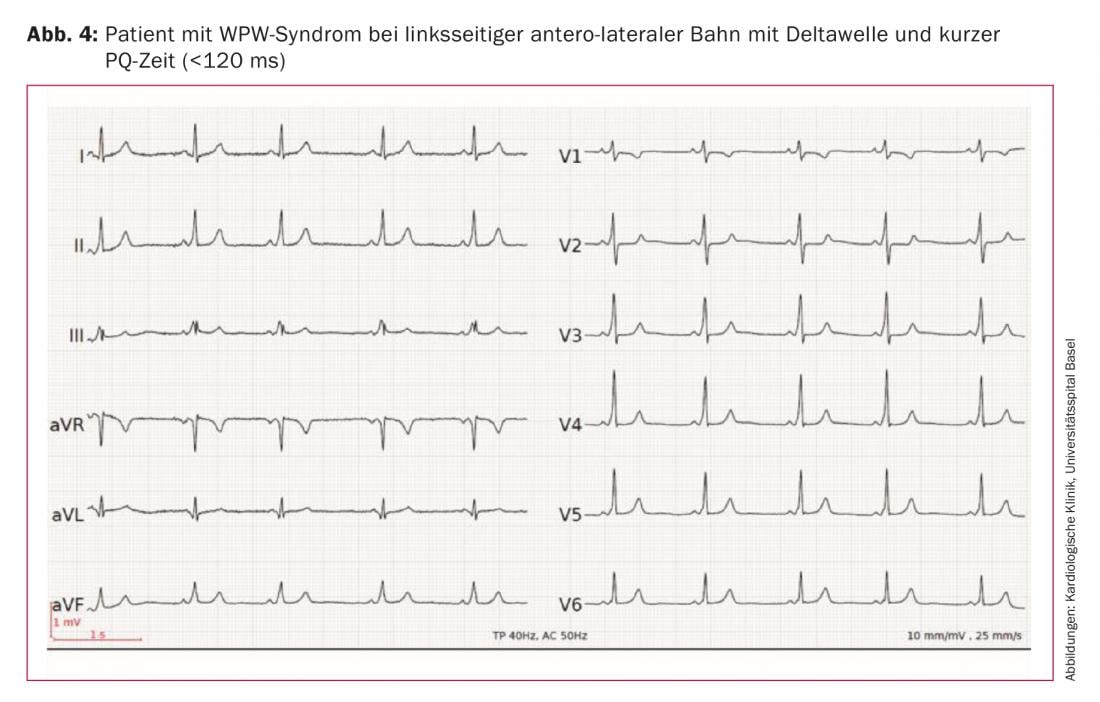
5. the resting ECG: after conversion to normocardial sinus rhythm, the resting ECG should be examined for signs of preexcitation. If the accessory pathway conducts anterograde and this leads to a shortening of the PQ time (<120 ms) and the appearance of a delta wave, preexcitation is present. If tachycardia is also present, it is referred to as Wolff-Parkinson-White syndrome (WPW syndrome). Figure 4 shows the resting ECG of a patient with WPW syndrome and left antero-lateral pathway. Important: If the accessory pathway conducts exclusively retrograde (“concealed pathway”), a normal ECG without delta wave is found at rest.
Therapy in the emergency ward
Therapeutically, vagal maneuver or adenosine administration is the first line of treatment for regular narrow complex tachycardia. Adenosine is administered intravenously, in flush, in 6-mg increments (half-life 1.5 seconds). Occasionally, repetitive administration at higher doses up to 18 mg is necessary [4]. The patient must be informed that the drug can have a very unpleasant effect during seconds (dyspnea, tightness, headache). Atrial fibrillation can be induced in up to 12% of cases and, in rarer cases, non-sustained ventricular tachycardia [4], so ECG monitoring is mandatory and a defibrillator should be available. An equally feared side effect is severe bronchoconstriction, so adenosine is contraindicated in patients with asthma and caution is advised in severe COPD. Alternatively, the cardiac calcium antagonists verapamil or diltiazem can be used here, although preexisting beta-blocker therapy is a relative contraindication (risk of developing AV block). Electrical cardioversion is reserved for patients with hemodynamic instability, which, however, is very rare in patients with supraventricular tachycardia.
Therapy in the cath lab
If vagal maneuvers and medications do not help, the seizures accumulate, become prolonged, and result in repeated emergency department or office consultations, an electrophysiologic study (EPU) is indicated. There, the diagnosis can be confirmed and subsequently radiofrequency ablation (RFA) of the “slow pathway” in AVNRT or the accessory pathway in AVRT can be performed with a high probability of success (>95%) [5,6]. Complications of EPU are rare and include local bleeding in the groin, vascular injury, or thromboembolic complications. Accessory pathways, which are in close proximity to the His bundle, pose an additional risk of damage to the AV node during ablation. There is also a slightly increased risk in left accessory pathways because a transseptal puncture must be performed in this case. Alternatively, pharmacological antiarrhythmic therapy is possible in patients with regular narrow complex tachycardia without preexcitation, which is extremely rarely used nowadays because of the high success rate of EPU/RFA. Evidence is available for the efficacy of drugs in the beta-blocker group, verapamil, and digoxin. In 30-60% of cases, these drugs reduce the frequency and severity of tachycardia [7], but complete suppression is extremely rare. Class Ic or III antiarrythmic drugs would theoretically be conceivable in the event of failure of AV-blocking medications, but long-term therapy is usually undesirable because of the side-effect profile.
Conclusion
In patients with narrow complex tachycardia, as in our patient in the case vignette, the clinic and 12-lead ECG already provide important clues for establishing the diagnosis. Initial evaluation in the emergency department or physician’s office should include the regularity and onset of tachycardia (on ECG or by history). Differential diagnoses of regular narrow complex tachycardia include AVNRT, AVRT, atrial tachycardia, and atrial flutter. Heart rate can provide further clues. With sudden onset, and absent or retrograde P waves, AVNRT is likely. The next step is a vagal maneuver or administration of adenosine under ongoing ECG monitoring. After termination of the arrhythmia, the resting ECG should be examined for evidence of preexcitation. An electrophysiological examination confirms the diagnosis, and subsequent radiofrequency ablation has a probability of success in curing the arrhythmia of >95%.
Take-Home Messages
- In patients with narrow complex tachycardia, defined by a heart rate ≥100 bpm (“beats per minute”) and a QRS duration of ≤0.12 seconds, clinic and 12-lead ECG already provide important clues for finding the diagnosis.
- Differential diagnoses of regular narrow complex tachycardia include AV nodal reentry tachycardia (AVNRT), AV reentry tachycardia (AVRT), atrial tachycardia, and atrial flutter. With sudden onset, and absent or retrograde P waves, AVNRT is likely.
- The response to vagal maneuvers or administration of adenosine is extraordinarily useful not only therapeutically but also diagnostically, so it is imperative that this be done with ongoing ECG monitoring. After termination of the arrhythmia, the resting ECG should be examined for evidence of preexcitation.
- An electrophysiological examination confirms the diagnosis. Radiofrequency ablation has a probability of success in curing the arrhythmia of >95%. Complications are extremely rare.
Literature:
- Delacrétaz E: Supraventricular tachycardia. N Engl J Med 2006; 354(10): 1039-1051.
- Link MS: Evaluation and Initial Treatment of Supraventricular Tachycardia. N Engl J Med 2012; 367(15): 1438-1448.
- González-Torrecilla E, et al: EGC diagnosis of paroxysmal supraventricular tachycardias in patients without preexcitation. Ann Noninvasive Electrocardiol 2011; 16(1): 85-95.
- Riccardi A, et al: Adenosine in the treatment of supraventricular tachycardia: 5 years of experience (2002-2006). American Journal of Emergency Medicine 2008; 26: 879-882.
- Kuck KH, et al: Radiofrequency current catheter ablation of accessory atrioventricular pathways. Lancet 1991; 337: 1557-1561.
- Calkins H, et al: Diagnosis and cure of the Wolff-Parkinson-White syndrome or paroxysmal supraventricular tachycardias during a single electrophysiologic test. N Engl J Med 1991; 324: 1612-1618.
- Winniford MD, Fulton KL, Hillis LD: Long-term therapy of paroxysmal supraventricular tachycardia: a randomized, double-blind comparison of digoxin, propranolol and verapamil. Am J Cardiol 1984; 54: 1138-1139.
Further reading:
- Arya A, et al: Differentiating atrioventricular nodal reentrant tachycardia from tachycardia via concealed accessory pathway. Am J Cardiol 2005; 95(7): 875-878.
- Orejarena LA, et al: Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol 1998; 31(1): 150-157.
- Katritsis DG, Camm AJ: Classification and differential
- diagnosis of atrioventricular nodal re-entrant tachycardia. Europace 2006; 8(1): 29-36.
- Page RL, et al: 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2016; 133(14): e506-74.
CARDIOVASC 2018; 17(3): 12-17











