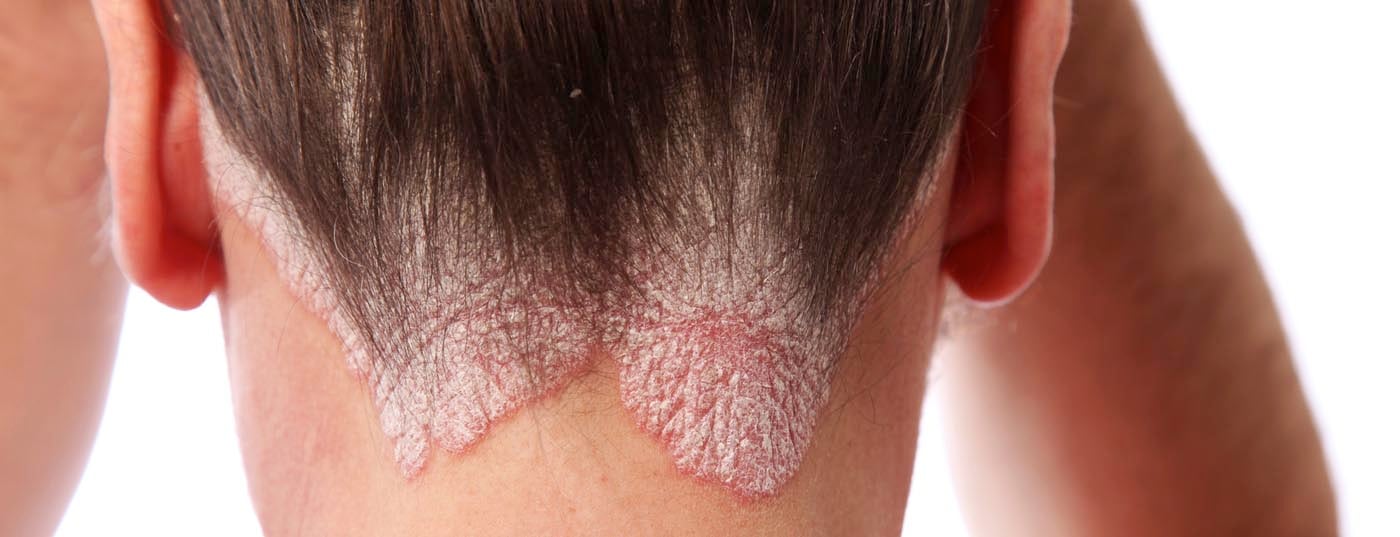
Recently, a large multinational study investigated the impact of psoriasis and psoriatic arthritis (PsA) on the lives of people with the condition. This showed that various areas of the disease situation require intensified attention and an increased need for action. For example, a high number of under-treated patients were found, as well as different assessments of the disease on the part of the physician and on the part of the affected person. So the hope is for new therapeutic options. In this context, the results of a large-scale phase III trial presented at the AAD Congress in Denver are interesting.
(ag) The MAPP study (“The Multinational Assessment of Psoriasis and Psoriatic Arthritis”) [1] examined disease-related quality of life (QoL), the doctor-patient relationship, unmet treatment needs, and patient satisfaction with current psoriasis and psoriatic arthritis (PsA) options. The aim was to find out to what extent the currently achievable therapy successes differ from the individual patient’s experience, which indeed still seems to be a major problem: The majority of patients (85%) would like to have better treatment options. Nearly 60% of PsA patients reported receiving no therapy. Also, the majority of psoriasis sufferers with 4% or more body surface area affected received no or only topical therapy. This is due in no small part to the fact that although many patients underwent traditional oral or biologic medication at one time or another, they very often had to stop it (57% with traditional oral medication and 45% with biologics). Reasons for this were mostly found in safety, tolerability and efficacy problems. Approximately 50% of patients with psoriasis and PsA reported that they found therapy with both traditional oral medications and biologics burdensome.
As expected, the study also showed a remarkable negative impact of psoriasis and PsA on QoL. It is crucial to note that physicians and patients obviously assess the severity of the conditions differently. For example, the majority of patients described itching as the most disturbing symptom, but according to the authors, this point is neglected in the medical assessment. To do this, they often focus on easily measurable disease elements such as lesion area and the number of affected joints. However, this does not adequately reflect the burden of disease, since, for example, patients with small-surface lesions on the hands and feet are also severely limited in terms of everyday life.
New therapeutic option on the horizon?
Carle Paul, MD, Toulouse, presented the 16-week results of the phase III randomized controlled trial ESTEEM 2 at this year’s AAD Congress in Denver [2]. In this study, apremilast (APR), an oral phosphodiesterase 4 (PDE4) inhibitor, was evaluated in 413 patients with moderate to severe psoriasis (“psoriasis area and severity index ” PASI ≥12, “body surface area” BSA ≥10%, and “static physician global assessment” sPGA ≥3). In this study, after randomization, 138 patients received placebo and 275 patients received APR 30 mg twice daily. This over a period of 16 weeks. This was followed by a phase in which all patients were treated with APR until week 32 and a randomized discontinuation phase until week 52.
Week 16 results: highly significant more patients achieved PASI-75 (28.8%) and PASI-50 (55.5%) on APR compared with placebo (5.8 and 19.7%, respectively, p<0.0001). The median/mean changes from respective baseline PASIs were -15.8/-18.0% for placebo and -50.9/-56.0% for APR. Significantly more patients achieved an sPGA score of 0 or 1 with APR (20.4%) compared with placebo (4.4%, p<0.0001). That is, APR enabled complete (0) or near-complete (1) decay significantly more often.
APR also showed significantly (p<0.0001) higher response rates to difficult-to-treat areas compared to placebo: Psoriasis on nails, scalp and also, but less significantly (p=0.0315), on palmoplantar zones responded well to APR. This is encouraging, according to Dr. Paul, because psoriasis at these sites severely affects chronic sufferers. In line with the MAPP study, this shows an improved benefit of psoriasis therapy.
The majority of observed adverse events, including nausea, diarrhea, nasopharyngitis, tension headache, and vomiting, were mild to moderate and rarely resulted in treatment discontinuation (by week 16 at 5.5%, which is comparable to the 5.1% on placebo). A flare (recurrence) or relapse of psoriasis was measured more frequently during the sixteen weeks on placebo (5.1%) than on APR (1.5%). Diarrhea and nausea on APR had the highest incidence in the first week and usually retreated completely within a month. Severe adverse events such as serious infections, malignancies, and cardiovascular events were consistent with previous APR trials.
Already, a separate analysis of ESTEEM 1 on the safety and tolerability of apremilast [3], also presented at the AAD Congress, had shown no new or unexpected adverse events compared with those seen in week 16 or earlier phase II trials.
Source: American Academy of Dermatology (AAD) Annual Meeting, March 21-25, 2014, Denver.
Literature:
- Lebwohl MG, et al: Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. Journal of the American Academy of Dermatology 2014; 70(5): 871-881.e30.
- Paul C, et al: Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients With Moderate to Severe Psoriasis: 16-Week Results of a Phase 3, Randomized, Controlled Trial (ESTEEM 2). AAD 2014 Poster #8412.
- Reich K, et al: Long-Term Safety and Tolerability of Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients With Moderate to Severe Psoriasis: Results From a Phase III, Randomized, Controlled Trial (ESTEEM 1). AAD 2014 Poster #8296.
CONGRESS SPECIAL 2014; 5(2): 9-10