Its heterogeneity has led to asthma being regarded as an umbrella term rather than a disease in its own right. It encompasses several aspects and features that can overlap, making asthma management difficult and requiring accurate phenotyping. But be careful: once a phenotype has been diagnosed, it does not necessarily remain set in stone – it can change over the course of a lifetime.
Nowadays, a basic distinction is made based on the extent of type 2 inflammation and differentiates between T2-high and T2-low asthma. Prof. Dr. Fabio L.M. Ricciardolo, Department of Severe Asthma and Rare Lung Diseases, University Hospital San Luigi Gonzaga, Turin, and colleagues [1] write that several phenotypes are subordinate to each of these two forms.
The classification of asthma phenotypes into T2-high and T2-low is currently based on atopic predisposition and at least one of the biomarkers elevated serum immunoglobulin E (IgE), high levels of fractionated exhaled nitric oxide (FeNO) and eosinophilia in the blood (B-EOS) and/or sputum (S-EOS).
T2-high asthma
T2-high phenotypes have a common immune-inflammatory response driven by Th2 lymphocytes (adaptive immunity) and ILCs (innate lymphoid cells) of group 2 (ILC2, innate immunity). The hallmarks of T2 inflammation are T2 cytokines such as IL-5, IL-4, IL-13, IL-9, prostaglandin D2 (PGD2) and eosinophils, the high expression of which can be detected in the airways (lumen or bronchial wall) and in the peripheral blood of patients. In fact, the term eosinophilic asthma is synonymous with T2-high asthma, includes both allergic and non-allergic phenotypes and affects about 50% of patients with severe asthma.
Allergic asthma develops after exposure to various aeroallergens that cause activation of dendritic cells (DCs). DCs in the subepithelial layer act as antigen-presenting cells and can recognize and process allergens. As soon as they are activated, the so-called early and then late phase of the allergic reaction occurs.
In addition to inflammation of the airways, T2-high asthma is accompanied by structural changes in the airways (remodeling). These changes include metaplasia of the goblet cells, which leads to increased mucus production and the resulting formation of mucus plugs. A recent study showed that the airways of patients with severe asthma and T2 inflammation have increased mucus plugs, with eosinophils playing a key role in the formation of the plugs.
Early onset allergic asthma
Typical triggers and symptoms of childhood asthma are allergens and other environmental stimuli such as viral infections, pollutants, oxidants and cigarette smoke. This creates an immune and inflammatory cascade, which is responsible for acute bronchoconstriction. This early onset ast hma (EOA) is usually characterized by atopy with the presence of circulating allergen-specific IgE and is characterized by the so-called “atopic march”, which is a progression from atopic dermatitis to allergic rhinitis and asthma.
EOA can last a lifetime. Studies looking at the determinants of this persistence pointed to maternal smoking during pregnancy, upper and lower respiratory tract infections, atopy, wheezing caused by rhinovirus infections and blood eosinophilia in early childhood as risk factors for lower lung function, asthma and severe asthma in school-age childhood, adolescence and young adulthood.
Late onset non-allergic asthma
Non-allergic asthma begins in adulthood and can be defined as late onset ast hma (LOA). Like allergic asthma, LOA is characterized by a pronounced T2 immune and inflammatory signature and is characterized by the absence of atopy and the resulting IgE signaling.
LOA can manifest in different degrees of severity, with the severe form leading to poorer lung function, more severe airway obstruction and increased eosinophilia despite treatment with high doses of inhaled corticosteroids (ICS), the authors write. High concentrations of ILC2 were found in the airways and peripheral blood of patients with severe non-allergic asthma characterized by uncontrolled eosinophilia in sputum and steroid resistance, indicating a mechanism possibly involving IL-33.
Analgesic asthma
Aspirin-exacerbated respiratory disease (AERD) occurs in close association with respiratory symptoms that develop rapidly after taking analgesics such as acetylsalicylic acid or a non-steroidal cyclooxygenase-1 (COX-1) inhibitor. Reactions to NSAIDs, asthma and nasal polyps are characteristics of the syndrome (Samter triad). In both adults and children, either allergic asthma or allergic rhinitis can manifest before analgesic hypersensitivity, but AERD is not considered an allergic disease because it does not produce specific IgE antibodies. About 8-26% of patients with CRS and nasal polyps have AERD at the same time, while the prevalence is about 7% in all asthma patients and twice as high in patients with severe asthma.
T2-low asthma
T2-low asthma includes all forms of asthma without the characteristic features of T2-high asthma. Based on the inflammatory profile of the airways, a distinction is made between neutrophilic (sputum neutrophilia with low eosinophilia), mixed (high sputum neutrophilia and eosinophilia) and paucigranulocytic (low sputum neutrophilia and eosinophilia) asthma. Low T2 asthma can vary in severity, with mixed and neutrophilic phenotypes tending to have higher severity and exacerbation rates. There are no validated molecules for biomarkers yet, although studies with blood/airway samples from asthma patients show encouraging results, according to Prof. Ricciardolo and colleagues.
The causes of neutrophilic airway inflammation include bacterial and viral infections, with the latter being the cause of asthma exacerbations. A higher frequency of exacerbations and a higher incidence of CRS and viral infections were observed in patients with neutrophilic inflammation.
In asthma patients exposed to high doses of ICS and/or long-term treatment with oral corticosteroids (OCS), the use of corticosteroids can lead to a non-eosinophilic phenotype. However, studies found that patients with a molecular phenotype, including patients with severe eosinophilic asthma, were more likely to take OCS than patients with a neutrophilic molecular phenotype. In addition, several bronchial biopsy studies showed a reduced or stable neutrophil status after corticosteroid treatment.
Smoker’s asthma
Tobacco smoke can trigger pathogenetic processes and lead to a phenotype associated with a number of features: accelerated deterioration of lung function, poor asthma control, more frequent exacerbations, lower quality of life, higher hospitalization rates and a higher risk of comorbidities such as cancer. Asthma in smokers has been reported to be associated with low T2 inflammation.
The steroid resistance associated with smoking and the exclusion of smokers with asthma from most clinical trials pose a major challenge in the treatment of this phenotype.
Obesity-induced asthma
Obesity-induced asthma usually has a severe form in which patients are often steroid-resistant. Changes in clinical and functional characteristics, such as airway hyperresponsiveness (AHR), can be improved by weight loss. Obesity is a pathological condition characterized by systemic inflammation involving macrophages. Macrophages are thought to be responsible for the inflammation of adipose tissue, which leads to the release of TNF-α, leptin and IL-6 into the bloodstream. 62% of obese asthma patients have low IL-6 plasma levels. One study showed that high IL-6 levels were associated with severe asthma and metabolic disorders in both obese and non-obese patients.
Asthma in older people
Age-related asthma is present when the disease first occurs after the age of 65. Such patients have a higher morbidity and mortality rate. There are many reasons for this, including comorbidities, inadequate awareness of asthma symptoms, potential cognitive decline and adverse effects of polypharmacotherapy. Ageing processes that change the structure and physiology of the lungs also have an effect on asthma and its development. Age-related changes include a narrowing of the peripheral airways, alveolar dilatation, increased stiffness of the chest wall, reduced strength of the respiratory muscles and reduced lung function. The exact pathophysiology of this phenotype is unclear, which makes it very difficult to treat, the authors write. Further studies are required to clarify the possible connection with corticosteroid resistance in older asthma patients.
Postmenopausal asthma
A higher proportion of boys are affected by asthma in childhood. This changes in adulthood, where the proportion of women is higher. In addition to genetic factors, this development is also attributed to hormones: High concentrations of oestrogen and progesterone can modulate the expression and activity of immune cells, inflammatory cytokines and glucocorticoids, which leads to the induction of a Th2 reaction with eosinophils and an increase in FeNO in the premenstrual phase. This probably contributes to the severity and worsening of asthma symptoms, exacerbations and hospitalizations during menstruation, perimenopause and pregnancy.
Studies on menopausal asthma have shown an increased risk of asthma in lean women taking hormone replacement therapy, as well as reduced lung function and increased asthma symptoms in postmenopausal women compared to premenopausal patients. Further research is needed to investigate the internal mechanisms of postmenopausal asthma.
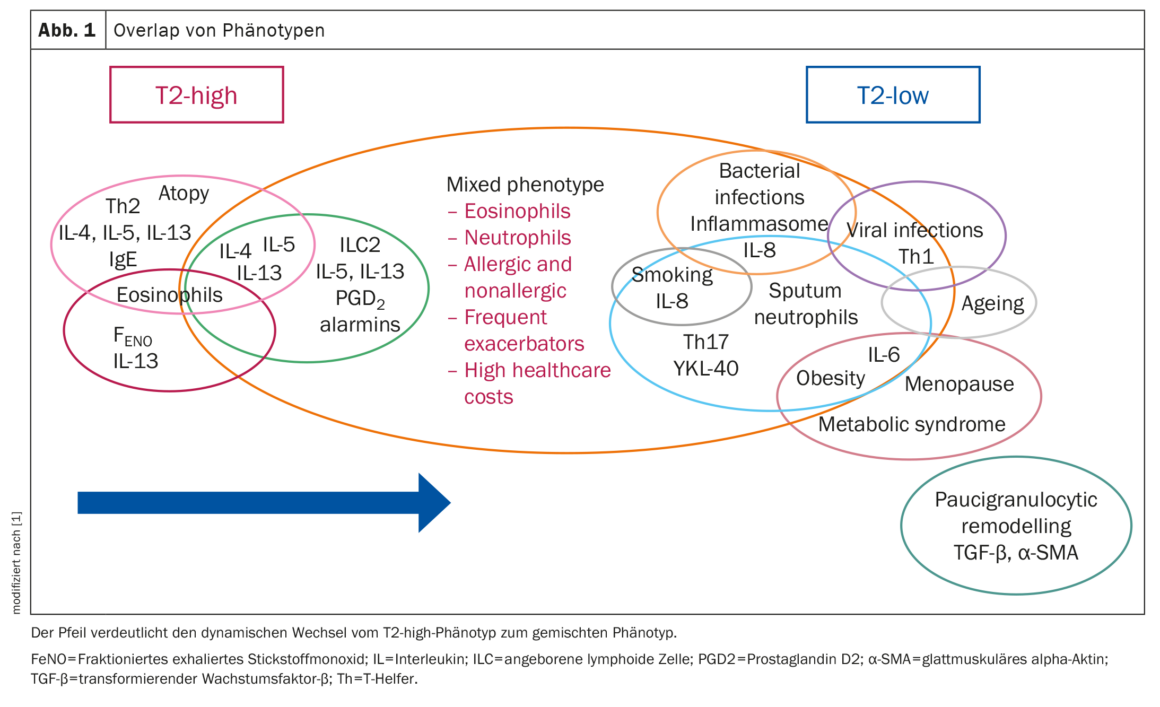
Overlap of the phenotypes
Identifying the asthma phenotype is crucial for the treatment of the disease, explain Prof. Ricciardolo et al. However, multiple molecular pathways may be intertwined and overlap between different phenotypes, which may result in unexpected clinical outcomes. Several studies have provided evidence of an interplay between pathways that were previously thought to be mutually exclusive (Fig. 1).
In one study, overlap was observed in >70% of patients, with combinations of T2-related, non-T2-related and mixed T2/non-T2 inflammation-related phenotypes, with the last group having the worst clinical outcomes. Overlaps of the T2 phenotype were detected in various combinations of allergic, eosinophilic and T2-high subtypes. The allergic phenotype was the most common single phenotype, but also the one that most frequently occurred simultaneously with other phenotypes. Persistent EOA and allergies were associated with the highest risk of developing COPD, while LOA and allergies were associated with a higher risk of multimorbidities, including diabetes, obesity and cardiovascular disease.
Asthma and related comorbidities may have common molecular mechanisms, common exposures or common genetic predispositions, the authors speculate. Comorbidities (COPD, GERD, cardiovascular disease), which are usually associated with a low T2 inflammation signature, can thus also develop in patients with an initially high T2 state.
Changes and possible evolution to a different phenotype may result in poor clinical control, steroid insensitivity and worsening of severity. When defining the asthma phenotype based on current biomarkers, physicians should therefore be more cautious and also look for overlaps between different phenotypes, especially in steroid-insensitive and difficult-to-treat asthma patients, the researchers conclude.
Literature:
- Ricciardolo FLM, Guida G, Bertolini F, et al: Phenotype overlap in the natural history of asthma. European Respiratory Review 2023; 32: 220201; doi: 10.1183/16000617.0201-2022.
InFo PNEUMOLOGY & ALLERGOLOGY 2024; 6(1): 36-38