Early detection, risk stratification, and tailored therapy of systemic sclerosis (SSc) are of high importance to counteract disease-related limitations and potentially life-threatening manifestations in a timely manner. Interstitial lung disease (ILD) is an organ manifestation of SSc associated with significant mortality. In addition to immunosuppressants, the antifibrotic drug nintedanib has been available in Switzerland for some time and tocilizumab was recently approved in the USA based on positive phase III results.
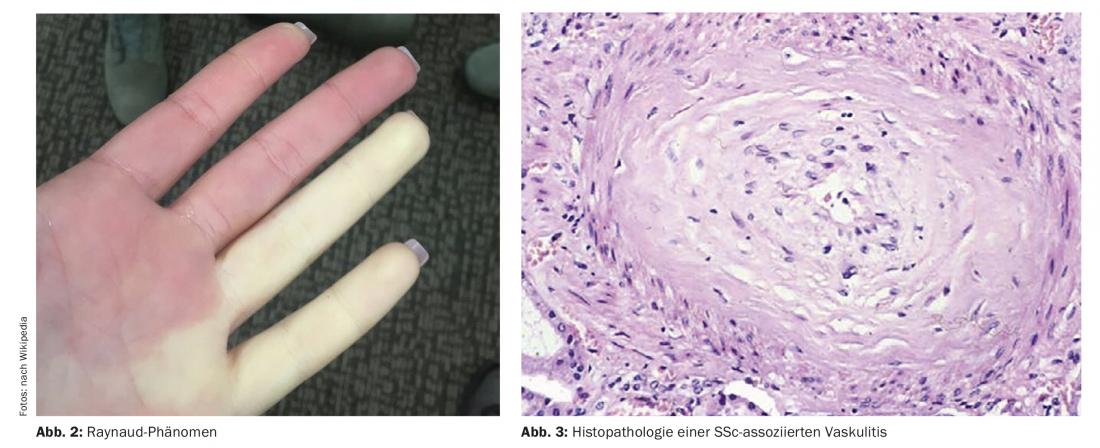
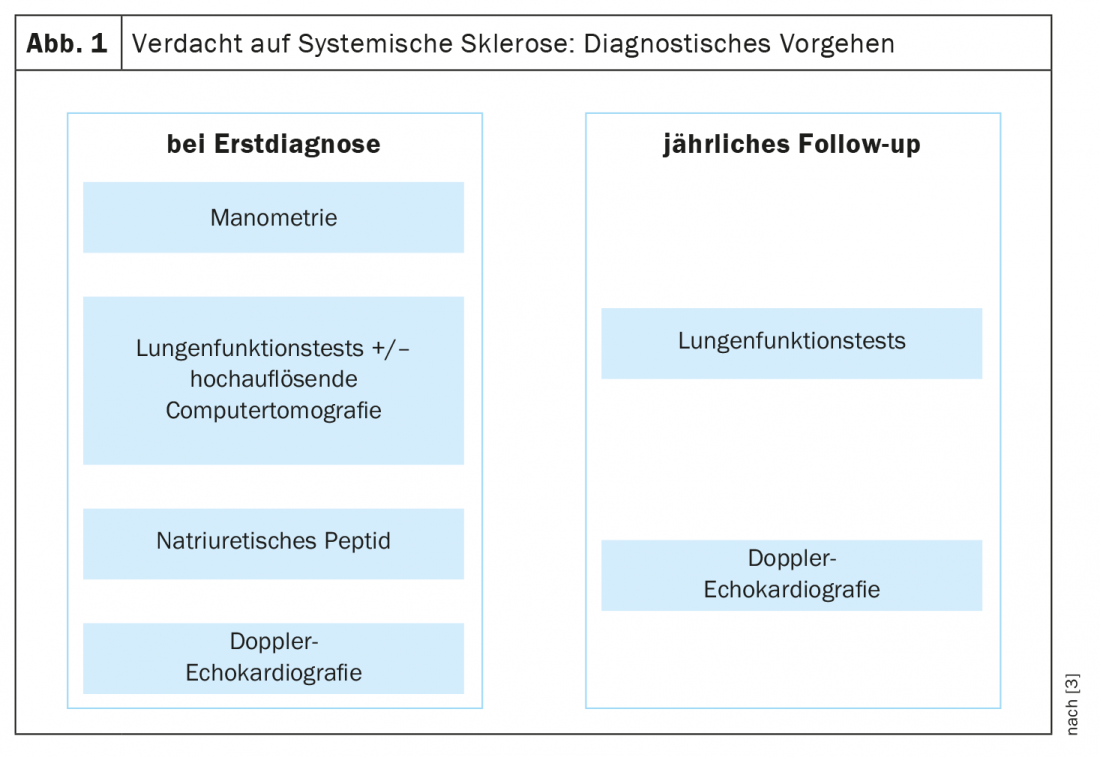
For the early diagnosis of systemic sclerosis, the VEDOSS criteria (“Very Early Diagnosis of Systemic Sclerosis”) were established several years ago [1,2]. Raynaud’s syndrome, finger swelling (“puffy fingers”), changes in nail fold capillaries, and detection of antinuclear autoantibodies (ANA) are predictive of systemic sclerosis (SSc), explained Dr. Hanna Grasshoff, Department of Rheumatology and Clinical Immunology, University Medical Center Schleswig-Holstein, Lübeck [1]. The classification criteria established in 2013 by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) are still valid [1,16]. The most common SSc-associated ANAs include anti-centromere Ak (ACA) and anti-topoisomerase Ak (ATA, Scl70). Recent empirical findings confirm that the VEDOSS criteria are appropriate for risk stratification of patients. This was also shown in a 2021 study published in the European Journal of Internal Medicine [3]. The speaker summarized the diagnostic procedure as follows (Fig. 1) : “One screens patients with esophageal manometry, pulmonary function tests, and if necessary high-resolution computed tomography if there are abnormalities, as well as natriuretic peptide and Doppler echocardiography” [1]. An additional ECG may be useful [1]. Annual pulmonary function tests and Doppler echocardiograms are recommended during the course. Regarding pulmonary arterial hypertension, including SSc-PAH, a new ESC/ERS guideline has been published in 2022 [17]. The previous target values have been modified and are now as follows: mPAP (mean pulmonary artery pressure) >20 mmHg, PAWP (pulmonary arterial wedge pressure) ≤15 mmHg, PVR (pulmonary vascular resistance) ≥ 2 WU (Wood* units).
* traditional unit of measurement of vessel resistances
“Making the most of “windows of opportunity
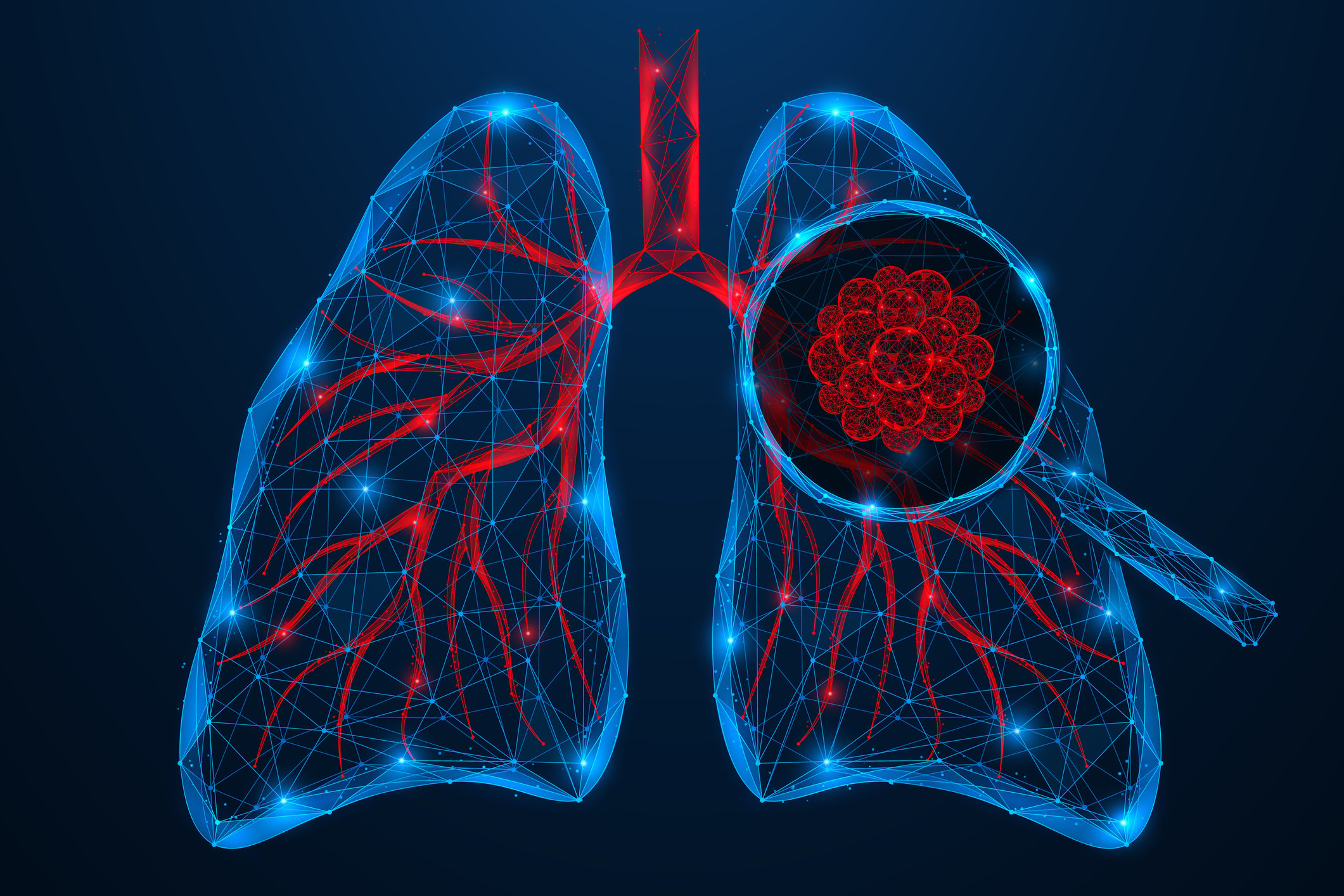
The distinction between limited-cutaneous SSc and diffuse-cutaneous SSc is still relevant. According to EULAR recommendations, the therapeutic approach depends on the organ involvement [5]: 1. Raynaud’s syndrome, 2. digital ulcers, 3rd SSc-PAH, 4. skin and lung manifestation, 5. renal crisis, 6. gastrointestinal manifestation. The two most commonly used drugs are cyclophosphamide and mycophenolate mofetil (MMF), based on two randomized-controlled trials with similar efficacy results [6,7]. In the Scleroderma Lung Study (SLS) II, cyclophosphamide and MMF achieved comparable efficacy, but MMF had the better long-term safety and tolerability profile (Fig. 4). Therefore, MMF is more commonly used in clinical practice. Of great interest, of course, is the question of what implications early diagnosis has for therapy. In a retrospective cohort study published in 2022, SSc patients who manifested diffuse cutaneous SSc or interstitial lung disease within 6 years of disease onset were divided into early and delayed intervention groups based on disease duration. In the former, treatment initiation occurred within ≤18 months since disease onset, and in the latter, only >18 months after [4]. Drug therapy options included cyclophosphamide, MMF, methotrexate, or tocilizumab. In the early intervention group, active disease decreased significantly from 79% to 42% (p=0.007), while the change in the delayed intervention group was not statistically significant (68% to 42%; p=0.11). Overall, the findings of this study support the notion that there is a “window of opportunity” for therapeutic options in SSc patients.
SSc-ILD – expanded therapeutic spectrum for life-threatening complication
For skin and lung manifestations, EULAR recommends the following drug options: Methotrexate, cyclophosphamide, autologous hematopoietic stem cell transplantation, possibly MMF, possibly azathioprine. Interstitial lung disease (ILD) in systemic sclerosis (SSc-ILD) is currently the most common disease-associated cause of death in patients with SSc [8]. The prevalence of ILD as a complication of SSc is approximately 50% in a recent population-based cohort [9]. The main drug therapy used so far has been immunosuppressants, with MMF being the most commonly used drug internationally [10,11]. From recent studies, nintedanib and tocilizumab may help slow the deterioration of lung function in SSc-ILD [12]. Nintedanib is an antifibrotic tyrosine kinase inhibitor used to treat lung tissue scarring in interstitial lung disease. In Switzerland, nintedanib (Ofev®) has been approved since 2020 for the treatment of interstitial lung disease associated with systemic sclerosis [12]. The recommended dosage is 150 mg twice daily, approximately 12 hours apart. Tocilizumab was approved by the U.S. Food and Drug Administration (FDA) for the treatment of SSc-ILD based on data from a Phase III trial [13].
Precision medicine points the way to the future
On autologous hematopoietic stem cell transplantation (ahSZT) in systemic sclerosis, a position paper of the working group stem cell therapy of the German Society of Rheumatology was published [14]. Accordingly, ahSZT is reasonable under the following conditions: maximum disease duration of 4 years, mRSS of at least. 15, internal organ involvement or other prognostically unfavorable factors, inadequate response to cyclophosphamide or MMF. SSc is a very heterogeneous disease. “We need long-term molecular stratification at different OMICS levels,” said Dr. Grasshoff [1]. This is where precision medicine comes in. “The goal would be to treat vasculopathy vasoactively, inflammation immunomodulatory, and fibrosis antifibrotic,” she said [1]. Studies correlating intrinsic gene signature with response to different drugs are a promising approach for a subgroup-specific targeted treatment strategy with an optimized benefit-risk profile.
Congress: German Rheumatology Congress
Literature:
- “Systemic sclerosis – a heterogeneous disease,” Dr. Hanna Grasshoff, German Rheumatology Congress, Aug. 31-Sept. 3, 2022.
- Minier T, et al: Ann Rheum Dis 2014; 73(12): 2087-2093.
- Gonzalez Garcia A, Callejas-Rubio JL: European Journal of Internal Medicine 97(Suppl 113); DOI:10.1016/j.ejim.2021.12.012.
- Yomono K, Kuwana M: Rheumatology (Oxford) 2022; 61(9): 3677-3685.
- Kowal-Bielecka O, et al: Ann Rheum Dis 2017; 76(8): 1327-1339.
- Tashkin DP, et al: NEJM 2006 (354): 2655-2666.
- Tashkin DP, et al: Lancet Respir Med 2016 (4): 708-719.
- Elhai M, et al: Ann Rheum Dis 2019; 78: 979-987.
- Hoffmann-Vold AM, et al: Am J Respir Crit Care Med 2019; 200: 1258-1266.
- Fernández-Codina A, et al: Arthritis Rheumatol 2018; 70: 1820-1828.
- Khanna D, et al: [abstract]. Arthritis Rheumatol 2018, 70 (suppl 9). https://acrabstracts.org/
- Drug Information, www.swissmedicinfo.ch, (last accessed Nov. 07, 2022).
- Khanna D, et al: Lancet Respir Med 2020; 8: 963-974.
- Alexander T, Burmester G: Journal of Rheumatology. Issue 5/2020.
- Schneider U, et al: Z Rheumatol 2021; 80: 868-878.
- van den Hoogen F, et al: Arthritis Rheum 2013; 65(11): 2737-2347.
- Humbert M, et al: ESC/ERS Scientific Document Group. EHJ 2022; 43(38): 3618-3731.
HAUSARZT PRAXIS 2022; 17(11): 16-17