Arteriovenous malformations (AVM) consist of congenital multiple direct connections between arteries and veins. Growth with resulting possible complications such as bleeding, pain and ulceration is almost 100% expected. The necessary multidisciplinary treatment of patients with arteriovenous malformations should be performed at centers specialized for this purpose. Arteriovenous malformations are usually chronic diseases, as they are rarely curatively treatable. Only if complete surgical removal is possible can AVM be treated curatively. For this, early correct diagnosis is essential. Treatment of surgically incompletely resectable AVM is complex and requires multiple interventions, with the primary therapeutic goal being symptom control. Interventional closure of the venous portions of the AVM is a very good therapeutic option.
Arterial malformations can be divided into arteriovenous malformations (AVM), arteriovenous fistulas (AVF), and arterial malformations, which include congenital stenoses or atresias. In 1982, Mulliken and Glowacki published their nomenclature of vascular anomalies, which was adopted by the International Society for the Study of Vascular Anomalies (ISSVA) [1]. This nomenclature also includes the differentiation of arteriovenous malformations. These are rare (prevalence approximately 0.15%) congenital changes in the arterial stromal pathway that are usually not diagnosed until childhood and adolescence.
Arteriovenous malformations are congenital, multiple, direct connections between arteries and veins. The absence of resistance vessels results in direct arteriovenous shunting. In current literature, the central portion of the AVM is referred to as the nidus, Latin for nest. In contrast to AVM, AVF is a pathological singular (often acquired) short circuit between an artery and a vein. Most AVMs are found in the head, usually intracerebrally. The cause of this is unclear, but it is thought that preexisting primitive shunts fail to close due to lack of apoptosis.
Most AVMs are caused by a spontaneous mutation. However, familial subgroups are also found in which genetic aberrations such as the RASA1 mutation have been detected [2]. Misdiagnosis is very common in AVM and often results in inadequate treatment.
AVM clinic
Small AVM often show only a reddish discoloration of the skin (Fig. 1), which is slightly overheated. In this case, they may resemble other vascular malformations, such as capillary malformation. Palpation may reveal a slight buzz, which is due to arteriovenous shunting, in which high flow velocities occur. There is usually no pain at this stage. With increasing size, AVM become more and more symptomatic and clinically evident due to the increase in vessel diameter with high volume load (Fig. 2). All congenital arterial malformations such as AVM, but also congenital stenoses, aplasias or atresias, may be present individually or be part of a syndrome.
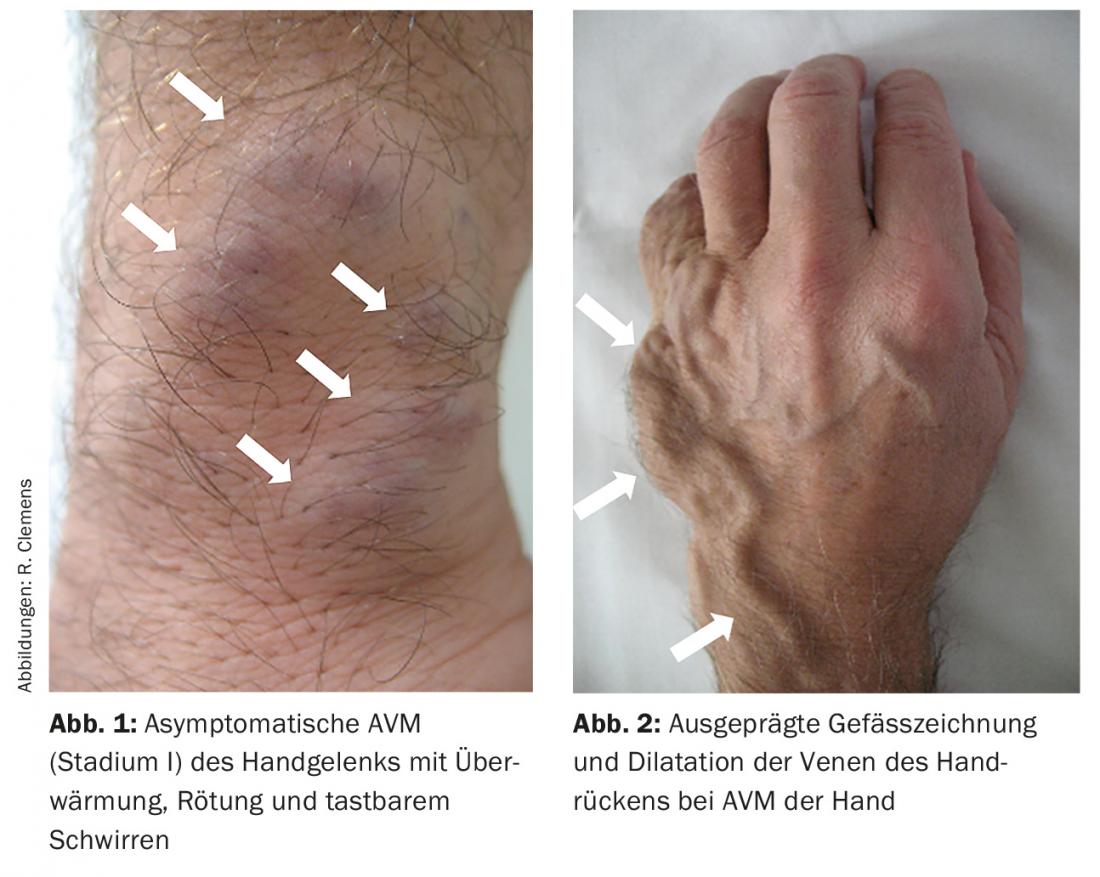
Complications of the AVM arise from its size with corresponding displacement effect and the resulting deformities. On the extremities, disturbed length growth (mostly proportioned overgrowth, more rarely undergrowth) as well as venous hypertension and, as a result of a steal phenomenon, deficient skin supply with ulceration may occur. Large AVM (as well as AVF) can lead to congestive heart failure due to the high shunt volume. Spontaneous arterial bleeding is described. Further complications result from the localization of the AVM – in intracerebral AVM, for example, epileptic seizures to fatal hemorrhage.
Classification
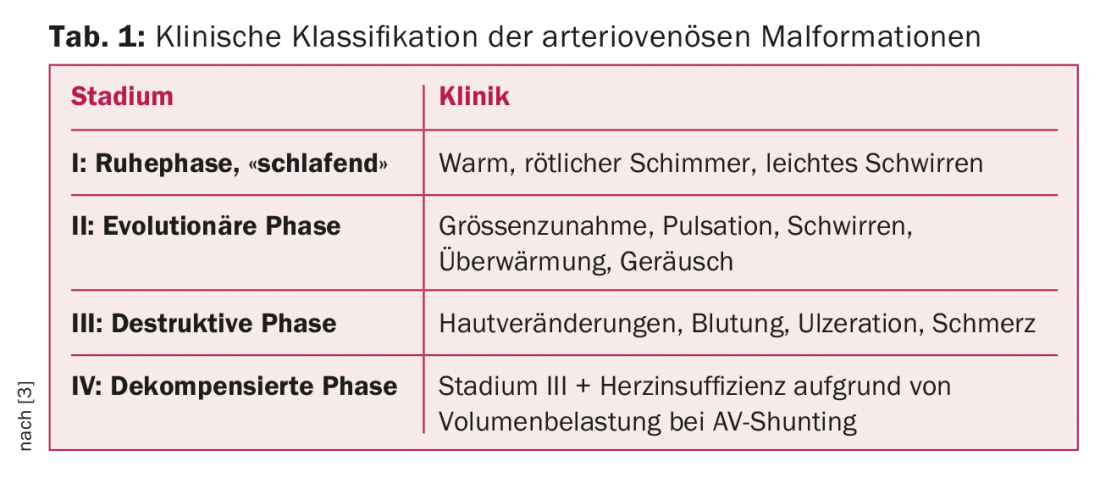
AVM can be clinically classified according to Schobinger (Table 1) [3]. Although progression to stage IV occurs in less than 10% of cases, nearly 100% of AVM become symptomatic during life, corresponding to a stage II-III according to Schobinger [4]. It is at these stages that the aforementioned complications occur. Treatment is then usually unavoidable.
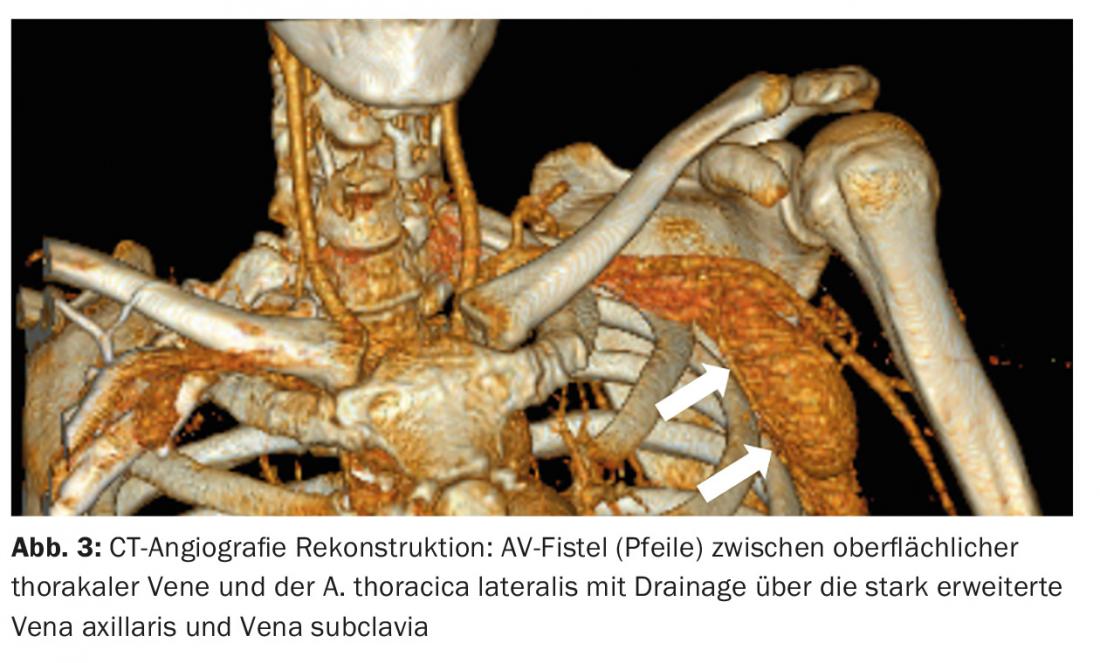
Angiographic classification, which primarily assesses the configuration of the feeding arteries and draining veins, is important for treatment strategy [5]. Chronic AVF may resemble AVM on imaging.
Diagnosis of arteriovenous malformations
Color-coded duplex sonography: In cases of clinical suspicion of extracranial arteriovenous malformation, color-coded duplex sonography (FKDS) is very well suited for initial evaluation because it does not require radiation exposure or potentially harmful administration of contrast media to the kidneys [6]. The B-scan can also distinguish between a vascular malformation and a vascularized tumor. Furthermore, the distinction between a fast-flowing malformation such as AVM and slow-flowing malformation such as venous malformation, which occurs much more frequently in percentage terms, is possible here. This has a decisive influence on further diagnostics and treatment. FKDS also allows noninvasive measurement of shunt volume, which is relevant to the indication for treatment and allows good monitoring after treatment.
Cross-sectional imaging: For further diagnostics, especially to show the extension of the malformation into the surrounding tissue, an MRI with contrast enhancement and, depending on the problem, also a CT with contrast is recommended. (Fig.3). In particular, the feeding arteries, the so-called “feeders”, but also the draining veins and surrounding involved structures should be assessed. However, the administration of contrast medium is not absolutely necessary for diagnosis.
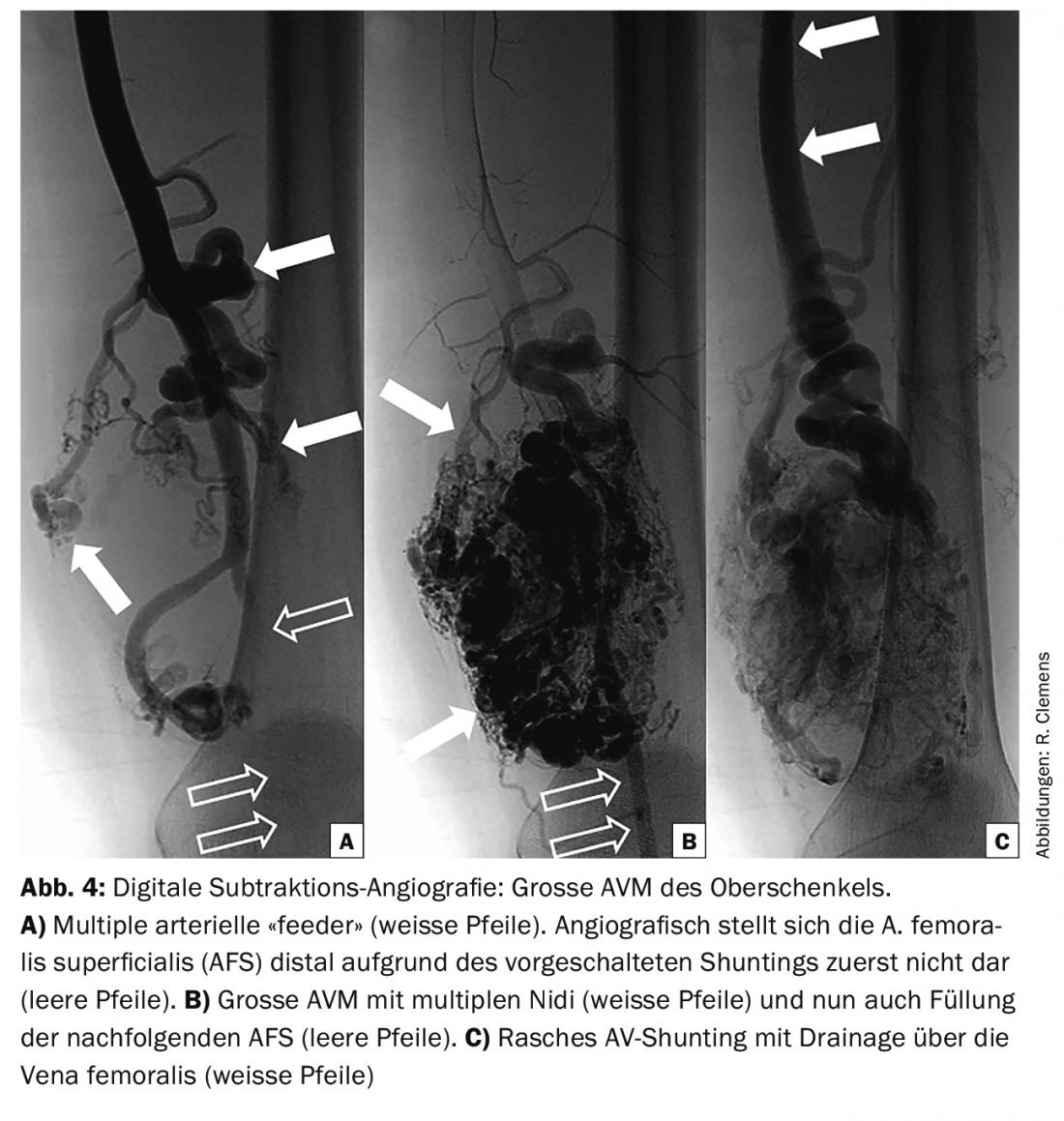
Angiography: Angiography is performed to plan treatment or as part of treatment and is only necessary in exceptional cases for purely diagnostic purposes. This involves assessment of the potentially treatable feeding and draining vessels and evaluation of hemodynamics and possible access routes. Angiography typically shows increased and enlarged arteries and rapid filling of draining veins, which are also often increased and dilated (Fig. 4) .
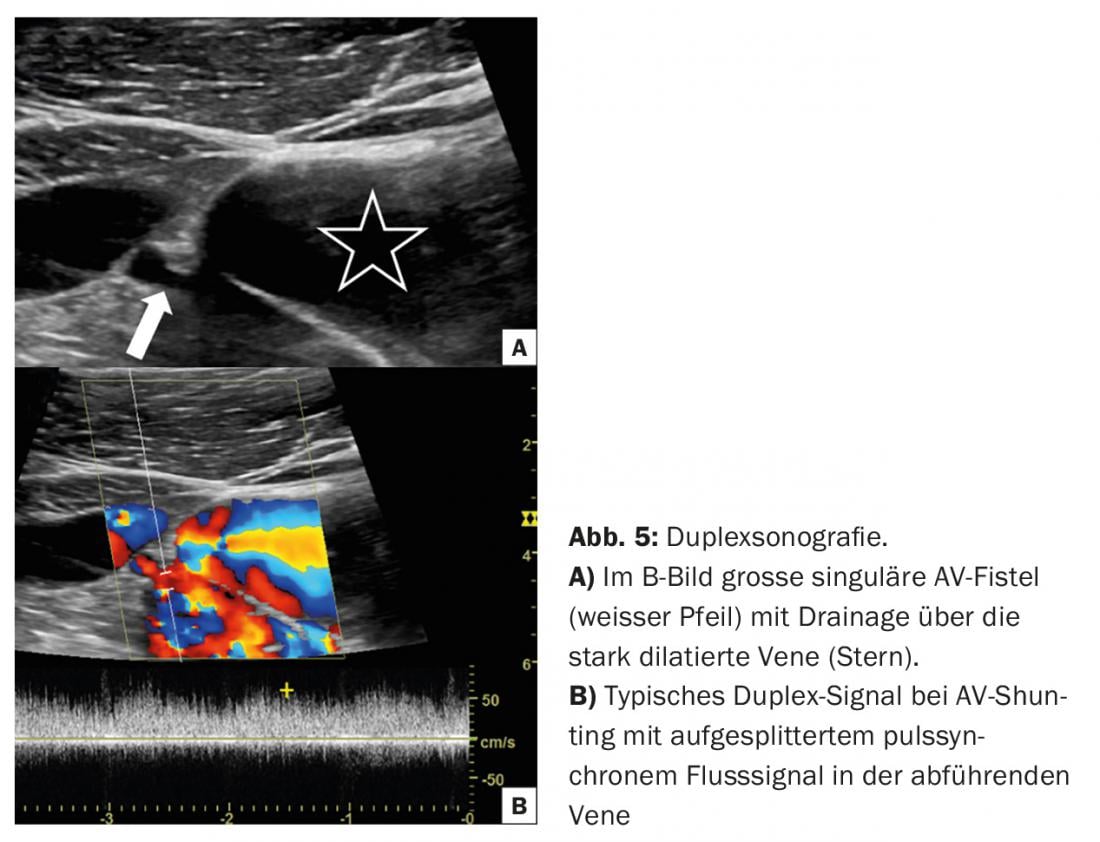
AV fistulas usually show a single connection between artery and vein, which is often easily visualized on FKDS (Fig. 5).
Interdisciplinary approach
Since congenital malformations are rare, multiple disciplines (angiology, dermatology, vascular surgery, neuroradiology, orthopedics, plastic surgery, radiology, visceral surgery, etc.) are often involved to develop an optimal treatment strategy. For this reason, interdisciplinary panels have formed at major centers to treat these patients.
Treatment of arteriovenous malformations
The treatment of arteriovenous malformations is complex. AVF, which usually consist of a single connection between an artery and a vein, can be treated in the majority of cases by interventional embolization or surgical ligation. Here, there is a high primary success rate without the risk of the procedure inducing growth of the malformation, as seen with AVM.
The much more common AVM are progedient in nearly 100% of cases, requiring treatment. In principle, excision with curative outcome is the treatment of first choice in AVM and should be performed as early as possible. However, since this is rarely possible, a conservative approach (with compression therapy where possible) may be indicated for the time being, especially if the AVMs are located in sensitive or highly visible areas. It should also be remembered that the deformity resulting from surgery may be more aesthetically detrimental than the AVM itself. Nevertheless, treatment is necessary early in the majority of cases, as only about one fifth of affected patients reach adulthood without the occurrence of complications [4].
Often the focus is on controlling the symptoms and size of the AVM, as complete excision is impossible. In stage II and III treatments, both transarterial embolization and surgical excision result in high rates of recurrence with incomplete treatment; this occurs in nearly 100% of cases for catheter-based procedures and in more than 80% for resections [4]. Because many patients have overgrowth or undergrowth of the affected extremities, orthopedic care is often required.
Interventional treatment options
To date, the leading interventional technique is arterial embolization of AVM with pure alcohol [7]. Here, the central part of the AVM, the nidus, is to be destroyed via the “feeder”, thus preventing shunting. However, complete elimination of AVM is rarely achieved. In addition, incomplete treatments often lead to a relevant growth spurt triggered by ischemia and consequent release of vascular growth factors. This happens especially when feeding arteries are embolized by means of coils or surgically interrupted (under the false assumption that this will “obliterate” the AVM). Coils consist of small platinum spirals that are advanced over the catheter in an elongated state and coil up in the vessel to form tight spirals that cause the blood to thrombose locally, thereby occluding the vessel. Often, this treatment now also occludes the proximal access route, making more distal embolization impossible. After transarterial embolization, previously invisible feeding arteries can often be visualized because they now fill more. This creates a situation where some arteries are occluded, but several others “open up” at the same time. Since transarterial treatment with alcohol, coils or tissue glue shows only limited success and often results in an increase in size during the course despite multiple embolizations, alternative interventional techniques are now more frequently used.
New interventional techniques
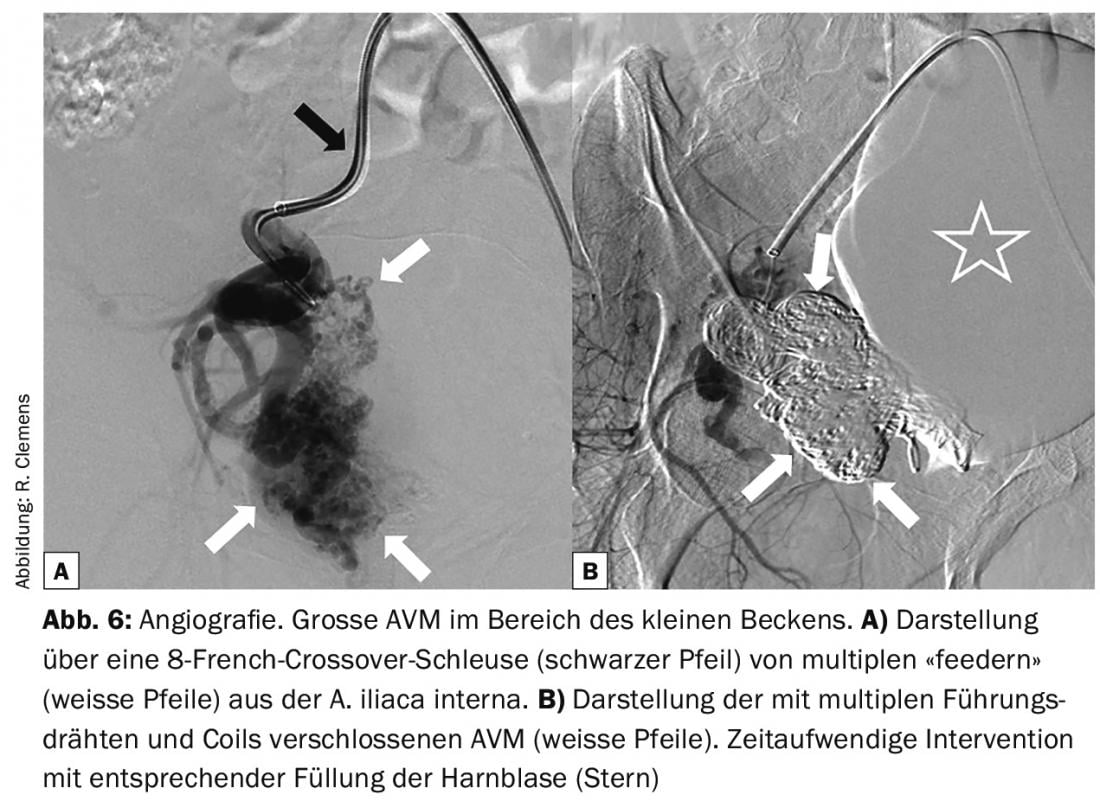
Interventional techniques include direct puncture and subsequent embolization of peripheral AVM with multiple coils or guidewires (Fig. 6) . Another method is aimed at the treatment of draining veins. This method relies on preventing arterial inflow via multiple “feeders” by occluding the draining veins. For this purpose, the veins are punctured by ultrasound and microcatheters are inserted. Over these, the veins are sealed with a variety of coils and a complementary tissue adhesive (n-butyl cyanoacrylate).
A promising alternative to the aforementioned techniques seems to be transvenous embolization with ethylene-vinyl alcohol copolymer (Onyx®) by “push-through” technique [8]. Here, in a time-consuming intervention, the Onyx® is pressed transvenously or transarterially through the nidus – thus closing both the central arterial and venous portions. Treatment of the central portions also avoids occlusion of potential arterial access routes for subsequent interventions.
However, because the arterial inflows of AVM are often diffuse and multiple connections may exist, which is equally true for the draining veins, the aforementioned treatments have limited feasibility. Here, the treatment goal is then “local” invasive treatment to stop bleeding or painful areas using transarterial embolization. For intracerebral AVM, arterial embolization is still the gold standard. The larger the lesions become, the more difficult and less successful the treatment usually is.
Drug treatment
Randomized-controlled trials of drug-systemic treatment of arteriovenous malformations are not available. However, there are case studies showing a beneficial effect of systemic administration of thalidomide [9]. A positive effect can also be observed with rapamycin, an mTOR inhibitor, both with treatment alone and periinterventionally to reduce treatment-induced growth spurt [10]. The successful administration of beta-blockers to limit size growth, as described in case reports, is controversial – but beta-blockers have an established place in infantile hemangiomas. Oral anticoagulation or the administration of antiplatelet agents is generally not indicated for “high-flow” vascular malformations that are not prone to thrombosis because of rapid flow.
Conflicts of Interest: The authors declare no financial support or other conflicts of interest related to this paper.
Literature:
- Mulliken JB, Glowacki J: Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plastic and reconstructive surgery 1982; 69: 412-422.
- Revencu N, et al: RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Human mutation 2013; 34: 1632-1641.
- Schobinger RA: [Diagnostic and therapeutic possibilities in peripheral angiodysplasias]. Helvetica chirurgica acta 1971; 38: 213-220.
- Liu AS, et al: Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plastic and reconstructive surgery 2010; 125: 1185-1194.
- Cho SK, et al: Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. Journal of endovascular therapy: an official journal of the International Society of Endovascular Specialists 2006; 13: 527-538.
- Paltiel HJ, et al: Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology 2000; 214: 747-754.
- Lee BB, et al: Consensus Document of the International Union of Angiology (IUA)-2013. Current concept on the management of arterio-venous management. International angiology: a journal of the International Union of Angiology 2013; 32: 9-36.
- Wohlgemuth WA, et al: The retrograde transvenous push-through method: a novel treatment of peripheral arteriovenous malformations with dominant venous outflow. Cardiovascular and interventional radiology 2015; 38: 623-631.
- Colletti G, et al: Adjuvant role of anti-angiogenic drugs in the management of head and neck arteriovenous malformations. Med Hypotheses 2015; 85: 298-302.
- Lackner H, et al: Sirolimus for the treatment of children with various complicated vascular anomalies. Eur J Pediatr 2015; 174: 1579-1584.
CARDIOVASC 2016; 15(2): 23-26