The therapeutic landscape of atopic eczema has recently been invigorated by innovative trends in topical and systemic treatment. The U.S. is ahead of the Europeans in terms of approval – as is so often the case.
The understanding of the pathogenesis of atopic dermatitis (skin barrier dysfunction and Th2-driven inflammation) is currently in a state of flux. Numerous innovative drugs are now being used to try to target various newly identified inflammatory pathways.
New topical therapy options
Crisaborole 2% Ointment was approved in the U.S. in late 2016 for the topical treatment of mild to moderate atopic dermatitis in patients two years of age and older. In addition to topical corticosteroids and calcineurin inhibitors, this provides another topical treatment option in the United States. The ointment, which is applied twice daily, contains a phosphodiesterase 4 inhibitor (PDE4 inhibitor crisaborole). In patients with atopic dermatitis, PDE4 activity is increased in inflammatory cells. Crisaborole inhibits PDE4 and its ability to degrade intracellular cyclic adenosine monophosphate. Thus, the release of proinflammatory cytokines can be prevented by medication and local inflammation can be reduced [1]. Currently in development are tapinarof (an agonist of the aryl hydrocarbon receptor for topical anti-inflammatory therapy and to improve epidermal barrier function) and topical JAK inhibitors, in addition to other PDE4 inhibitors.
Topical crisaborole effective and well tolerated in the short and long term
Two randomized, vehicle-controlled, multicenter, double-blind studies involving a total of 1522 patients between the ages of two and 79 years (86% between two and 17 years) demonstrated the efficacy and safety of the new topical therapy for 28 days [1]. The ISGA score (Investigator’s Static Global Assessment) was used to measure efficacy, which includes five grades (0 = healed, 1 = almost healed, 2 = mild, 3 = moderate, 4 = severe), assessing erythema, induration or papule formation, and oozing or crusting. At baseline, among the 1016 patients in the crisaborole group and the 506 patients in the vehicle group, moderate disease expression (ISGA grade 3) and in 38.5% a mild expression (ISGA grade 2) was found. Treatment success at day 29 was considered to be ISGA grade 0 (healed) or grade 1 (almost healed) with improvement of at least two grades since study entry. The crisaborole group resulted in treatment success significantly more often than the vehicle group (in 32.8% of patients vs. 25.4% in the first study AD-301, and in 31.4% vs. 18.0% in the second study AD-302) [1]. The significant “vehicle effect” has also been observed in other clinical trials in atopic dermatitis when active topical formulations were compared with the skin barrier function enhancing emollients (vehicles) [1]. Both skin findings and pruritus improved more rapidly with crisaborole ointment than with vehicle alone. The long-term safety of topical crisaborole therapy was evaluated in a 48-week open-label study in 517 patients following the two 28-day studies [2]. Reported adverse events were usually (in 93%) not related to crisaborole therapy. There was not often a possible association, e.g., pain (burning, stinging) or infection at the application site, worsening of atopic dermatitis with intermittent crisaborol therapy, nasopharyngitis, or upper respiratory tract infections. There was no evidence for safety concerns (e.g., regarding laboratory values, serious infections, neoplasms). None of the skin side effects feared with topical corticosteroids (telangiectasia, atrophy) were observed [2]. Because systemic absorption of crisaborole is low and degradation to inactive metabolites is rapid, the risk of systemic side effects is low [1].
Dupilumab as a new systemic therapy option
Much more is currently happening in the systemic treatment of atopic dermatitis than in topical therapy. The speaker even spoke of a “systemic therapy revolution”. Efforts to develop innovative systemic medicines have already been rewarded in the US with the approval of the IL-4/IL-13 blocker dupilumab in adults with moderate to severe atopic dermatitis inadequately controlled by topical treatment. This human monoclonal antibody is injected subcutaneously and binds to the common receptor subunit (IL-4R-α) of IL-4 and IL-13 on different immune cells, thereby inhibiting the signaling triggered by the two inflammatory Th2 cytokines IL-4 and IL-13. IL-4 and IL-13 are the two most important cytokines in the regulation of IgE synthesis and in the generation of atopic inflammation.
Two 16-week, randomized, placebo-controlled phase III trials, SOLO 1 (671 patients) and SOLO 2 (708 patients), evaluated the efficacy of 300 mg dupilumab subcutaneously every week and every two weeks, respectively, compared with placebo [3]. At 16 weeks, dupilumab achieved a 75% improvement in the Eczema Area and Severity Index (EASI75) in a significantly greater proportion of patients compared with placebo in both dosing regimens, e.g., in SOLO 1 with dupilumab weekly in 52.5% and with dupilumab every two weeks in 51.3% and with placebo in 14.7%, respectively. Dupilumab also provided rapid and sustained relief of pruritus [3].
Successful treatment with systemic dupilumab and topical corticosteroid.
In adult patients with moderate to severe atopic dermatitis, the long-term LIBERTY AD CHRONOS study evaluated the efficacy and safety of dupilumab (both dosing regimens) in combination with moderate-strength topical corticosteroids versus placebo injections (together with topical corticosteroids) for 52 weeks [4]. After 16 weeks, treatment in the group with dupilumab every week 300 mg subcutaneously together with topical corticosteroid therapy was successful in 64% of patients (EASI75), with the other therapy regimen (every two weeks) in 69%, and with placebo significantly less often in 23%. EASI75 response was also comparable at one year (64% and 65% vs. 22%, respectively). An EASI90 response was achieved at 52 weeks with both dupilumab dosing regimens in 51% and in the comparison group in 16% [4]. The treatment was also well tolerated in the long term. Puncture site reactions and conjunctivitis occurred more frequently in the two dupilumab groups than in the comparison group.
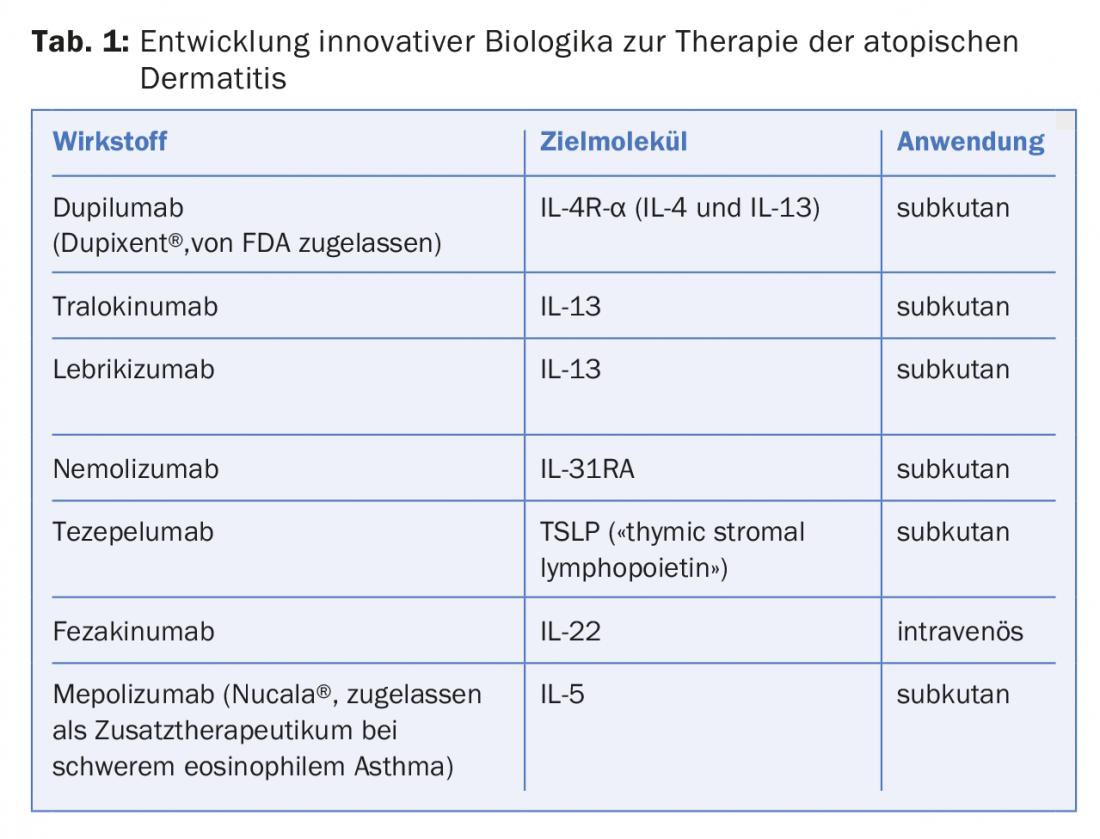
The current list of other drugs being developed for systemic therapy of atopic dermatitis is very long. It includes biologics targeting various cytokines or their receptors, as well as oral small molecule inhibitors to suppress inflammation or pruritus.
Some biologics currently being developed for the treatment of atopic dermatitis can be found in Table 1.
Source: 26th EADV Congress, September 13-17, 2017, Geneva.
Literature:
- Paller AS, et al: Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016; 75: 494-503.
- Eichenfield LF, et al: Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol 2017; 77: 641-649.
- Simpson EL, et al: Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335-2348.
- Blauvelt A, et al: Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287-2303.
DERMATOLOGIE PRAXIS 2017; 27(5): 43-44











