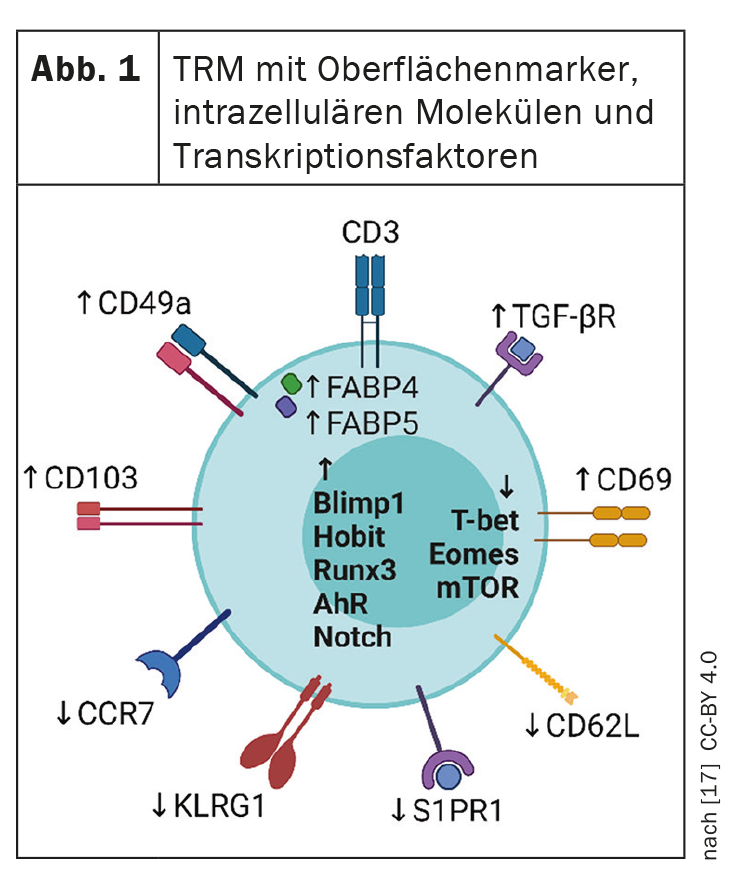
Immunological memory plays an important role in the pathogenesis of psoriasis. Increasingly, researchers are focusing on tissue resident memory T-cells (TRM cells). This is because the persistence of TRM cells in healed psoriatic lesions is one explanation for the recurrence of lesions at the same site after discontinuation of system therapy.
Modern highly effective systemic psoriasis therapies convert lesional skin to a state without visible clinical manifestations. However, once therapy is discontinued, psoriatic skin lesions often reappear in the previously affected areas. This suggests that residual disease activity persists in the area of apparently healed lesions, predisposing affected individuals to recurrence [2]. Tissue resident memory t-cells (TRM cells) ( Fig. 1) are part of the adaptive immune system and store information about antigens previously encountered by the immune system. TRM cells are thought to play a key role in the reactivation of latent inflammation and thus in long-term disease progression (box) .
CD8+ TRM cells as biomarkers of psoriasis activity?
A topical question is whether psoriasis can be gotten rid of by a “hit hard and early” approach, explained Prof. Wolf Henning Boehncke, MD, Hôpitaux Universitaires de Genève [1]. Several studies are investigating whether early intervention with biologics can have a lasting impact on the course of psoriasis and prevent the development of psoriatic arthritis or cumulative life course impairment (CLCI) [3–7]. Another question is whether TRM cells can be used as a biomarker for psoriasis activity, Prof. Boehncke said [1]. In a review article published in 2022 by Puig et al. the current state of knowledge is shown [8]. TRM cells can be divided into CD8+ and CD4+ subsets. CD8+ TRM cells are localized in the epidermis where they play a critical role in immune responses, CD4+ TRM cells are less well characterized and may be involved in protective immunity against bacterial and mycotic infections in the skin [9,10]. The majority of CD8+ TRM cells express the cell marker CD103. CD103 is the αE-subunit of αEβ7. E-cadherin is a ligand for integrin αE(CD103)β7. Integrin-αEβ7-E-cadherin interactions play a role in the organization of the epithelium of psoriatic skin as well as the organ-specific localization of T lymphocytes in the epidermis [11,12].
|
Psoriatic patients have more CD8+ TRM cells even in non-lesional skin The CD8+ TRM cells produce the proinflammatory cytokines IL-17 and IL-22 in the active state, express the IL-23 receptor, and are reactivated by IL-23. Vo et al. reported that CD8+ TRM cells are enriched in both lesional and non-lesional skin of psoriatic patients compared to normal skin [15]. This suggests that these TRM cells promote reactivation of latent inflammation and thus recurrence of lesions. Therefore, novel treatments for psoriasis that aim to achieve sustained remission of the disease should target depletion of pathogenic CD8+ TRM cells, researchers concluded [16]. |
Relevance for long-term modification of the course of the disease
In the future, it is hoped that biologics will be able to sustainably modify the pathophysiology of psoriasis by modulating TRM cells. Preliminary evidence suggests that inhibition of IL-23 inhibits TRM cell activation and thereby suppresses systemic inflammation in the long term. In the ECLIPSE study, analysis of psoriatic lesion biopsies showed that the number of CD8+ TRM cells in efflorescences was reduced to the level of non-lesional skin after 24 weeks of treatment with guselkumab [13,14]. The GUIDE study is investigating the hypothesis that early treatment with guselkumab may reduce the accumulation of TRM cells in the skin and thereby positively influence the long-term course of the disease [18].
Congress: Skin Inflammation & Psoriasis International Network
Literature:
- “Pathogenetics: psoriasis vs atopic dermatitis”, Plenary Session 4, Prof. Dr. Wolf Henning Boehncke, SPIN, Congress of the Skin Inflammation & Psoriasis International Network, 07.07.2022.
- Clark RA: J Invest Dermatol 2011; 131: 283-285.
- Gisondi P, et al: Expert Rev Clin Immunol 2020; 16: 591-598.
- Gisondi P, et al: Ann Rheum Dis 2022; 81: 68-73.
- Acosta Felquer ML, et al: Ann Rheum Dis. 2021 Ann Rheum Dis 2022; 81(1): 74-79.
- Von Stülpnagel CC, et al: J Eur Acad Dermatol Venereol 2021; 35: 2166-2184.
- Iversen L, et al: J Eur Acad Dermatol Venereol 2018; 32: 1930-1939.
- Puig L, et al: Br J Dermatol 2022; 186(5): 773-781.
- Chen L, Shen Z: Cell Mol Immunol 2020; 17: 64-75.
- Ho AW, Kupper TS: Nat Rev Immunol 2019; 19: 490-502.
- Watanabe R, et al: Sci Transl Med 2015; 7:279ra39.
- Pauls K, et al: J Invest Dermatol 2001; 117: 569-575.
- Warren RB, et al: J Eur Acad Dermatol Venereol 2021; 35: 919-927.
- Mehta H, et al: J Invest Dermatol 2021; 141: 1707-1718.
- Vo S, et al: Br J Dermatol 2019; 181:410-12.
- Armstrong AW, et al: Br J Dermatol 2020; 182: 1484-1487.
- Vu TT, et al: J Clin Med 2021; 10(17): 3822.
- Eyerich K, et al: BMJ Open 2021; 11(9): e049822.
DERMATOLOGIE PRAXIS 2022; 32(4): 20 (published 8/21-22, ahead of print).