Anxiety, sleep disorders, and depressive symptoms can cause great distress if left untreated. Therefore, it is important to take therapeutic measures even in the case of minor complaints. Many patients have reservations about synthetic psychotropic drugs because they fear side effects and symptoms of dependence. Especially for this target group, a well-tolerated drug based on a standardized lavender oil extract may be an interesting therapeutic option. A new study shows that the dependence potential of this anxiolytic and mood-enhancing substance is extremely low.
Anxiety disorders are among the most common psychiatric complaints and, even in subclinical manifestations, can lead to significant impairment of quality of life [1–3]. According to epidemiological data, the prevalence of generalized anxiety disorder (GAS) in developed countries is around 5% [4–6]. The leading symptom of GAS is excessive apprehension and constant worry [7]. In addition, physiological features such as restlessness, sleep disturbances, muscle tension, digestive disorders occur, which is related to dysfunctional activity of the sympathetic nervous system. Developing comorbid depression is also common in the setting of GAS.
This phytotherapeutic calms without being addictive
Guideline-based synthetic psychotropic drugs such as Selective Serotonin Reuptake Inhibitors (SSRIs), Selective Norepinephrine Reuptake Inhibitors (SNRIs), and tranquilizers are very effective, but especially regarding the anxiolytics benzodiazepine and pregabalin, a potential for dependence has been reported several times in clinical trials [1]. A well-tolerated herbal alternative for the treatment of restlessness and anxious mood is Silexan®. Studies have shown similar treatment effects with GAS as with lorazepam or paroxetine [8,9]. In controlled clinical trials, no sedative effects, dependence or withdrawal symptoms were reported after discontinuation with regard to Silexan® [1].
However, a human study to systematically investigate a possible dependence potential of Silexan® has been lacking. To fill this gap, a Phase I study was designed and conducted in 2015 at Syneos Health’s research facility in Toronto, Canada.
New study shows: Risk of dependence is extremely low with Silexan®.
To investigate substance habituation effects of Silexan® in single dose, a randomized, double-blind crossover study was conducted. Silexan® was compared intraindividually with lorazepam and placebo in healthy non-dependent subjects who occasionally consumed substances with depressant effects on the central nervous system (CNS) as stimulants. The choice of the benzodiazepine lorazepam as a comparator is based on the fact that Silexan® showed comparable treatment effects in patients with GAS in a previous randomized trial [8].
For the crossover study, 40 healthy female and male study participants were randomized to the experimental conditions. The age range was 18-54 years, and BMI ranged from 18.0-29.9 kg/m2. At the end of the study, evaluable data were available from a total of 34 subjects; the dropout rate was 15%. An inclusion criterion was experience in the use of substances with sedative effects on the central nervous system (CNS) in the history, at least 10× during the whole life and at least 1× within 12 weeks before the screening date of the study. This was chosen as an inclusion criterion because it is a risk group for developing dependence on active substances with sedative effects and substance use experience was beneficial for rating effects [1]. Exclusion criteria included somatic and mental illness, in particular substance dependence (including alcohol, excluding nicotine and caffeine) within the last 12 months and participation in substance withdrawal in the past history.
The implementation of the study included eight face-to-face appointments distributed over four phases: Screening, Qualification, Treatment, Follow-up. During the treatment phase, study participants received a single dose of placebo, lorazepam 2 mg, lorazepam 4 mg, Silexan® 80 mg, or Silexan® 640 mg at each of the five three-day clinic visits. Randomization was performed using Williams Square design. Within 24 h after substance intake, pharmacodynamic and -kinetic studies and evaluation of safety were performed. The study participants gave a rating on a visual analog scale of 0-101 (VAS) on their subjectively perceived condition as well as on the effects of the substances taken. The primary endpoint was subjective ratings of drug-liking.
Liking effect with Silexan® not greater than with placebo
Median individual maximum “liking” scores of 51 points were observed with both placebo and Silexan® 80 mg and 640 mg. 50 points corresponds to a neutral assessment on this scale. Under lorazepam 2 mg and 4 mg, these scores were 76 and 80.5 points, respectively. For Silexan® and placebo showed a slight increase in mean “liking” scores between 1 and 2.5 hours after ingestion above the neutral value of 50 points to mean peak values of 52.9 ± 12.4 points for Silexan® 80 mg, 58.6 ± 17.7 points for the 640 mg dosage, and 53.5 ± 5.5 points for placebo. Under lorazepam, mean “liking” scores reached individual maxima at 2.9 and 4.3 hours after ingestion for 2 mg respectively 4 mg with mean peak values at 66.9 ± 20.4 and 69.4 ± 19.7 points for 2 mg respectively 4 mg, and these values were still above the neutral value of 50 12 hours after ingestion. That the “liking” of the phytotherapeutic was not systematically distinguishable from placebo by the study participants is shown by the fact that the median difference between both doses of Silexan® and placebo was 0 (Table 1). In Figure 1 are the intraindividual comparisons between the different dosages of Silexan® and lorazepam compared with placebo. The intraindividual differences in the maximum values of the habituation effect (“liking”) between lorazepam and Silexan® were similar to those between lorazepam and placebo.
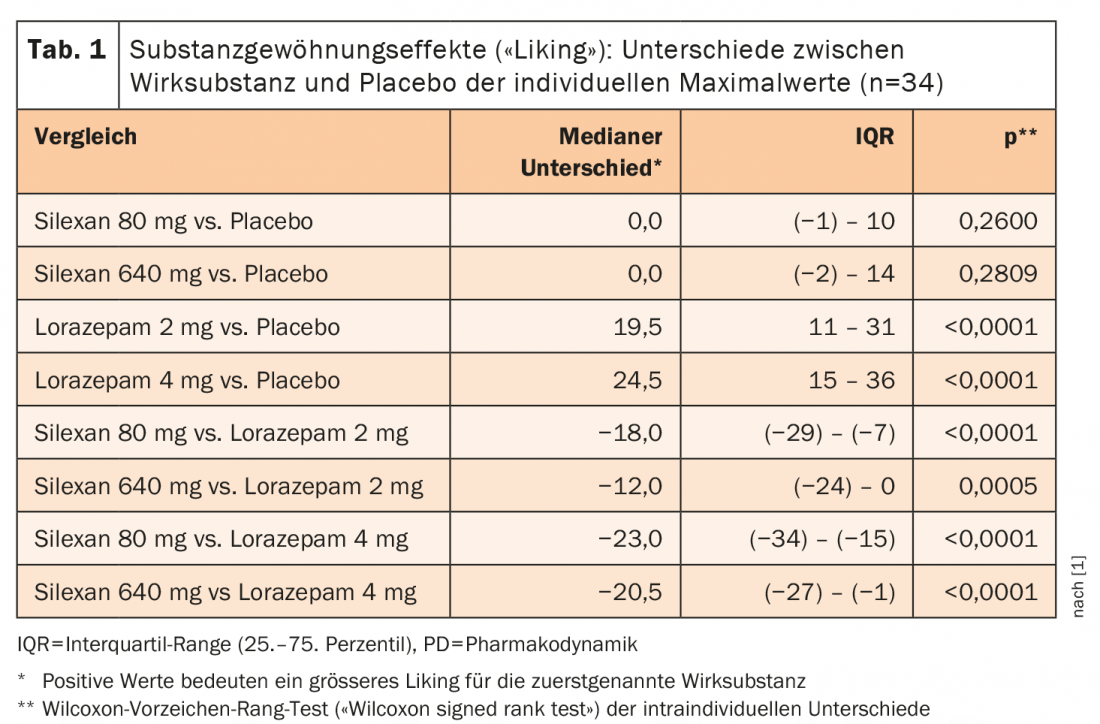
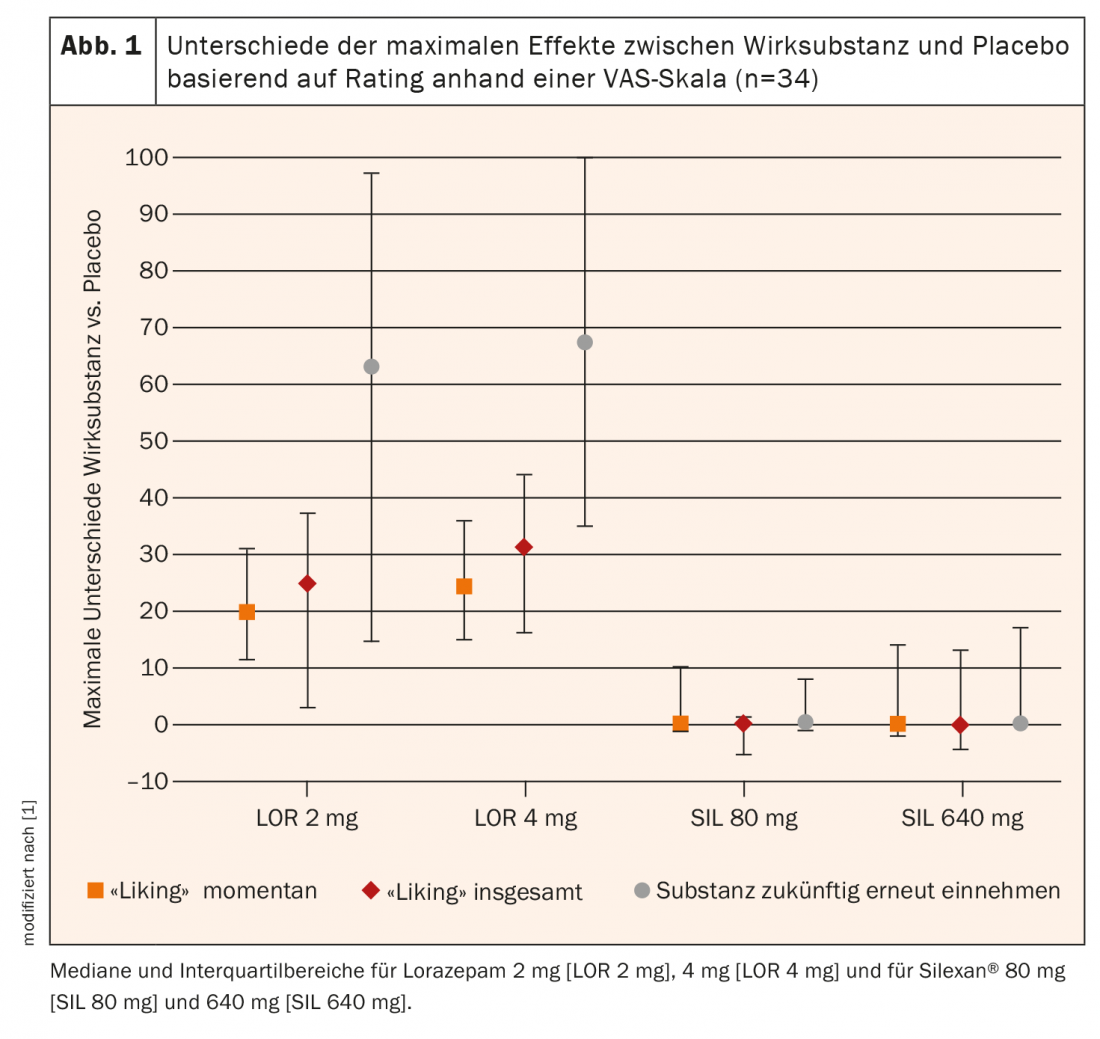
In summary, there was no evidence of dependence potential of Silexan® in this study, either at the recommended therapeutic dosage of 80 mg or at eight times the recommended dosage of 640 mg. In contrast to lorazepam, the rating values of the “liking” effect for Silexan® were consistently in the neutral range of the bipolar scale, i.e., between 40 and 60 points, which corresponds to the typical rating pattern for placebo substances [10].
Literature:
- Seifritz E, et al: No Abuse Potential of Silexan in Healthy Recreational Drug Users: A Randomized Controlled Trial. International Journal of Neuropsychopharmacology 2020, pyaa064, https://doi.org/10.1093/ijnp/pyaa064
- Karsten J, et al: Course and risk factors of functional impairment in subthreshold depression and anxiety. Depress Anxiety 2013; 30: 386-394.
- Möller HJ, et al: Efficacy of Silexan in subthreshold anxiety: meta-analysis of randomised, placebo-controlled trials. Eur Arch Psychiatry Clin Neurosci 2019; 269: 183-193.
- Ruscio AM, et al: Cross-sectional Comparison of the Epidemiology of DSM-5 Generalized Anxiety Disorder Across the Globe. JAMA Psychiatry 2017; 74(5): 465-475.
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders,5th ed. (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Zanni GR: Anxiety Disorders: Real Disease, Real Treatment. PharmacyTimes 2014, March 14, www.pharmacytimes.com
- ICD-10-GM-2018 Classification. www.icd-code.de
- Woelk H, Schläfke S: A multi-center, double-blind, randomised study of the lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine 2010; 17: 94-99.
- Kasper S, et al: Lavender oil preparation Silexan is effective in generalized anxiety disorder-a randomized, double-blind comparison to placebo and paroxetine. Int J Neuropsychopharmacol 2014; 17: 859-869.
- Setnik B, et al: Measurement of drug liking in abuse potential studies: a comparison of unipolar and bipolar visual analog scales. J Clin Pharmacol 2017; 57: 266-274.
InFo NEUROLOGY & PSYCHIATRY 2021; 19(1): 32-33.
HAUSARZT PRAXIS 2021; 16(2): 30-31