The occurrence of pain as a result of stroke presents a pain medicine challenge. Although the knowledge of pathophysiological mechanisms of neuropathic pain after stroke has increased significantly in recent years, the success of drug, neurosurgical, interventional, and other therapeutic approaches is currently still limited. A multimodal therapeutic approach is therefore mandatory. This article aims to provide an overview of diagnostic and therapeutic aspects of this disease entity.
Chronic moderate to severe pain is a common health care problem in Switzerland with a prevalence of 16%, noting that few patients are treated by pain specialists and approximately half receive inadequate pain management [1]. The presence of pure neuropathic pain is also common, with a prevalence ranging from 3.3% in a survey of the general population in Austria [2] to 7% in the general population in France [3].
The incidence of stroke in Europe is 1.1 million per year, with a projected increase to approximately 1.5 million per year by 2025, due to the increase in the proportion of older people in the population [4]. Chronic pain associated with stroke is observed in 11-55% of all stroke patients [5]. Common types of pain that occur after stroke can include central neuropathic pain, nociceptive shoulder pain, painful spasticity and also tension headaches. Central neuropathic pain occurs in 8% of all patients who have suffered a stroke [6]. Nociceptive shoulder pain is reported between 30% and 40% in stroke patients with sensory and motor deficits, shoulder subluxations, and limited range of motion [7]. Musculoskeletal pain can also occur in the back and lower extremities, especially in the knees and hips [8] (Table 1).

Central pain after stroke
The definition of neuropathic pain according to the International Association for the Study of Pain (IASP) assumes a lesion or disease affecting the somatosensory system [9]. To diagnose neuropathic pain, the IASP diagnostic criteria should be used to search anamnestically for pain that corresponds to a neuroanatomically circumscribed distribution and for evidence of a relevant lesion or disease of the peripheral or central nervous system. Clinical examination should demonstrate positive and negative sensory signs, corresponding to a circumscribed neuroanatomically plausible distribution and consistent with the pain area. In addition, instrumental diagnostics should demonstrate the presence of a lesion or disease of the somatosensory system [9].
In the English-language literature, central neuropathic pain after stroke is referred to as central poststroke pain (CPSP). In the absence of pathognomonic signs for CPSP, the Klit et al. following the IASP diagnostic criteria mentioned above, only recently proposed specific diagnostic criteria for CPSP [10]. Accordingly, the required criteria for the diagnosis of CPSP are pain in a body region that corresponds with a lesion of the central nervous system. The history should indicate that a stroke has occurred and, accordingly, the onset of pain should have occurred afterward. Furthermore, confirmation of a central nervous system lesion by imaging studies (e.g., CT or MRI of the skull) is required, or the presence of negative or positive sensory signs, compatible with a body region, should be demonstrated to correspond with a central nervous system lesion.
Because it is a clinical diagnosis of exclusion, other causes of pain such as nociceptive or peripheral neuropathic pain should be excluded or unlikely. The diagnosis may be supported by supporting criteria, such as the absence of a primary relationship of the pain to movement (e.g., of the extremities), the absence of signs of local inflammation, or signs of local tissue pathology other than a nociceptive cause of pain. A typical description of pain may include the indication of burning, tingling, pressing, pins and needles, sharp stabbing, shooting, constricting, cold pain, electrifying pain, although other descriptions are also observed. The presence of allodynia or dysesthesia to touch or cold in the pain area may provide additional clues (Table 2).

Clinical characteristics of neuropathic CPSP.
In the review paper by Klit [10] typical clinical characteristics of CPSP are summarized. The distribution of the pain area may affect only a small area, e.g., only the hand, or it may affect extensive areas of the body, up to and including hemiparesis. Also, the face and trunk may be omitted. Patients with a so-called lateral medulla oblongata infarct and resulting Wallenberg syndrome may also present with facial pain on the affected side and trunk and extremity pain on the opposite side (crossed syndrome). Periorbital pain is also frequently reported. Hemiparesis pain often occurs after a thalamic lesion. Sensory negative and positive phenomena are also typical in CPSP as in other neuropathic syndromes. Thermal disturbances, especially of the sensation of cold or also disturbances of the sensation of pain or disturbances of the pinprick or spike perception occur in more than 90% of the cases. Negative symptoms regarding touch or vibration occur less frequently. Positive phenomena such as evoked pain from thermal (especially cold stimuli) or mechanical stimuli (pinprick stimuli), but also allodynia to touch stimuli are common.
Central pain after stroke not only typically occurs after a thalamic lesion (“thalamic pain”) but also is reported after hemorrhage and ischemia in other areas of the brain [11]. Our own data show the occurrence of central pain after stroke in patients with brainstem infarcts, medullary lesions, and lesions of the insular cortex. In this context, very different causes were found regarding the genesis of stroke, such as microvascular or embolic cerebral infarction, cerebral infarction after vasospasm due to subarachnoid hemorrhage, or intracerebral hemorrhage [12].
The time between the onset of stroke and the onset of CPSP can be variable (immediate onset or onset of pain after years), but onset of pain within a few months is most typical [13]. In a prospective study of 16 patients [6], CPSP occurred in ten patients within the first month, in another three patients within one to six months, and in three patients after six months. Therefore, a late onset of CPSP should raise differential diagnostic considerations of other causes of pain or even the occurrence of a new stroke.
Role of instrumental diagnostics
According to the diagnostic criteria of the IASP mentioned at the beginning, imaging diagnostics of the brain should be performed, meaningfully an MRI to visualize the brain structures. If a lesion corresponding to the pain is detected, no further diagnosis is required. In individual cases, a lesion that is only very small may not be detectable on imaging. If the clinic is typical (history of stroke and evidence of corresponding negative and/or positive phenomena), a diagnosis of CPSP can be made. In addition, neurophysiological evidence of a lesion may be attempted. Somatosensory-evoked [14] and laser-evoked potentials [15], but also Quantitative Sensory Testing [16] can help to reveal a lesion. Our own data show pathological findings on laser-evoked potentials and quantitative sensory testing in most cases of patients with central neuropathic pain after stroke of different etiologies [12].
Mechanisms of pain development of neuropathic pain after stroke.
The recent review by Klit et al. [10] gives a good overview of possible pain mechanisms in central pain after stroke. Neuronal hyperexcitability at the level of the brain plays a significant role in the pathophysiology of centrally generated neuropathic pain. Factors that may promote neuronal hyperexcitability include glial activation with release of chemokines and inflammatory substances, weakening of inhibitory GABA-ergic systems of the thalamus, imbalance between the lateral and medial spinothalamic tracts due to a lesion there, or deafferentation of sensory input. Consequences of such mechanisms may include evidence of spontaneous activity in the ventrocaudal nucleus of the thalamus or alteration of cerebral blood flow in the thalamus. Additional mechanisms such as disruption of cortico-thalamo-cortical excitatory loops or cortical reorganization and also other mechanisms are discussed.
Therapeutic approaches for CPSP
Chronic pain after stroke can lead to a decreased quality of life that affects mood, sleep, and social functioning [17]. In general, the so-called multimodal pain therapy with somatic, physical and psychotherapeutic procedures is superior to monodisciplinary therapy for chronic pain syndromes. In this regard, as well as the various possible causes of pain outlined above, the treatment of post-stroke pain should always be interdisciplinary and multimodal. Especially in patients with neuropathic pain after stroke, psychological treatments such as coping strategies and behavioral therapies can lead to pain improvement [17].
Regarding nociceptive pain, according to the musculoskeletal pathology, mainly orthopedic (e.g. therapy of shoulder pathologies), physiotherapeutic (e.g. treatment of muscular pain factors) and occupational therapy measures are useful. In addition, therapy of painful spasticity with oral baclofen may be necessary. In special cases, the application of an intrathecal spasticity pump may be useful.
Drug therapy approaches: Drug therapy approaches for neuropathic pain after stroke have been evaluated in international guidelines such as the guideline of the Neuropathic Pain Working Group of the International Pain Society (NeuPSIG) [18] or the guideline of the European Federation of Neurological Societies (EFNS) [19]. The available drug therapy options are limited. It is possible that the multitude of pathophysiological mechanisms mentioned above at different neuronal levels is responsible for the often poor response to drugs available for the treatment of neuropathic pain after stroke. For a large number of the mechanisms mentioned, no therapy is yet available.
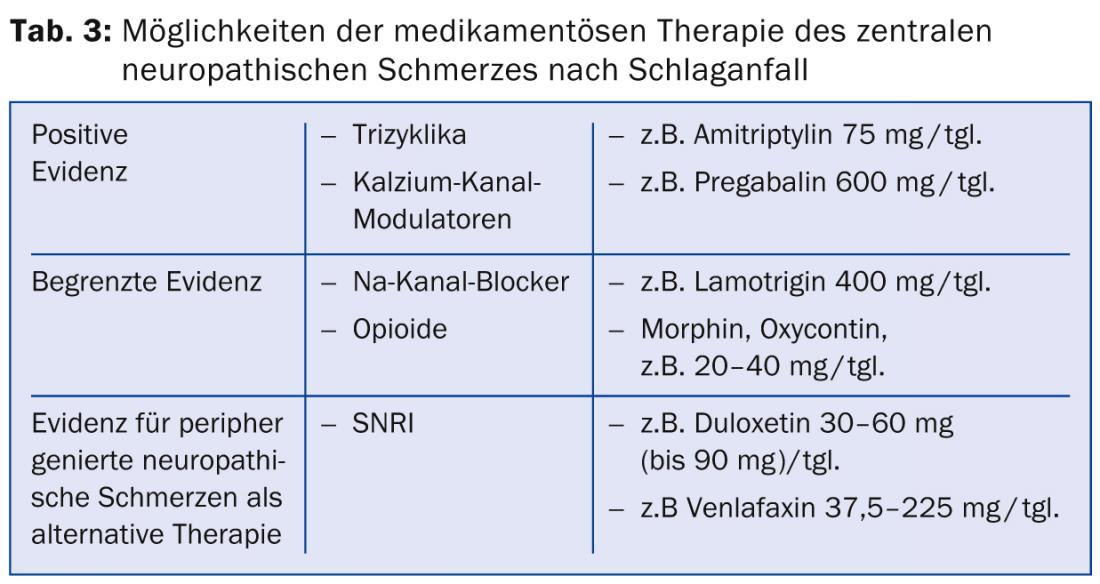
There are few randomized controlled trials for the treatment of central neuropathic pain after stroke. Tricyclics and calcium channel modulators are considered first-line drugs. Positive trial data are available for amitriptyline at the 75 mg dose [20]. The “number needed to treat” (NNT), i.e., the number of patients to be treated to achieve a 50% reduction in pain, was reported here as 1.7. Moderate side effects such as fatigue and dry mouth were observed.
Regarding anticonvulsants of the calcium channel modulator group, a study in patients with central neuropathic pain showed significant efficacy of pregabalin with an NNT of 4.0 [21]. Side effects included dizziness, cognitive impairment, fatigue, and nausea. Furthermore, the antiepileptic drug lamotrigine from the sodium channel blocker group showed moderate efficacy with good tolerability in a study of central pain after stroke [22]. In other neuropathic pain disorders, the efficacy of lamotrigine is not clear, so in general its efficacy is considered unclear [23]. A study with carbamazepine at a dose of 800 mg/tgl. showed no efficacy in neuropathic pain after stroke.
In a study in neuropathic pain of various etiologies, administration of oral opioids showed a mean pain reduction of 23%, although the number of patients with neuropathic pain after stroke was low, ten out of 81, and seven of them dropped out of the study because of side effects [24]. Studies with intravenous administration of morphine, lidocaine, and propofol showed improvement in poststroke pain during infusion. Subsequent oral maintenance therapies with morphine and mexiletine were poorly tolerated due to side effects [25–27].
If sufficient pain reduction cannot be achieved with the above medications, it is recommended to resort to first- and second-choice medications for the treatment of peripherally generated neuropathic pain [18]. These include, as first-line medications, the selective serotonin and norepinephrine reuptake inhibitors (SNRIs) such as duloxetine and venlafaxine, and for second-line medications, the highly potent opioids such as MST, Oxycontin, and other opioids (Table 3).
Neurosurgical and interventional therapeutic approaches: As shown, drug therapy options for neuropathic pain after stroke are limited and therefore often unsatisfactory. According to Dworkin et al. [23], invasive methods may be attempted after exhaustion of individual conservative or combined therapeutic procedures. The current data regarding neurosurgical and interventional therapeutic approaches have been compiled in recent reviews [10,28,29]. Because the evidence for these procedures is limited, they should be reserved for experienced treatment centers.
Repetitive transcranial magnetic stimulation (rTMS) represents a neuromodulatory, noninvasive procedure. Here, the effect is moderate and short-lasting, although serious side effects are unlikely to occur. Repeated treatments of the motor cortex with rTMS can show significant pain improvement [30,31]. In addition, this therapy can be used as a predictor of response to epidural motor cortex stimulation [32].
The efficacy of epidural motor cortex stimulation for central pain after stroke showed a pain reduction of approximately 45-50% after one year [28,33]. Severe complications are rarely reported. Perioperatively, epileptic seizures, infections, additionally technical problems may occur.
Deep brain stimulation (“DBS”) may be considered as another invasive therapeutic procedure. Here, the sensory thalamus (posterior ventral nerve) or the periventricular gray is stimulated. Efficacy has been reported to range from 25% to 67% [34,35]. In general, the effectiveness cannot be clearly quantified, so that further studies are called for here [28].
A new therapeutic method for patients with neuropathic pain including central neuropathic pain after stroke is the treatment with high intensity focused ultrasound (“HIFUS”), which is applied transcranially, non-invasively, to thermally ablate a circumscribed area of the centrolateral thalamus, which can lead to pain relief [36].
Gunther Landmann, MD
Dr. Emmanuelle Opsommer
Literature:
- Breivik H, et al: European Journal of Pain 2006; 10(4): 287-287.
- Gustorff B, et al: Acta Anaesthesiol Scand 2008; 52(1): 132-136.
- Bouhassira D, et al: Pain 2008; 136(3): 380-387.
- Truelsen T, et al: Eur J Neurol 2006; 13: 581-598.
- Jönsson AC, et al: J Neurol Neurosurg Psychiatry 2006; 77: 590-595.
- Andersen G, et al: Pain 1995; 61: 187-193.
- Lindgren I, et al: Stroke 2007; 38: 343-348.
- Kuptniratsaikul V, et al: Am J Phys Med Rehabil 2009; 88: 92-99.
- Treede RD, et al: Neurology 2008; 70(18): 1630-1635.
- Klit H, Finnerup NB, Jensen TS: Lancet Neurol 2009; 8(9): 857-868.
- Weimar C, et al: Cerebrovasc Dis 2002; 14: 261-263.
- Landmann G, Stockinger L, Opsommer E: Central post-stroke pain explored by laser-evoked potentials and quantitative sensory testing: A multiple case study. 30th International Congress on Clinical Neurophysiology (ICCN). Berlin, Germany, 2014.
- Tasker R: Central pain states. In: L JD, editor. Bonica’s management of pain Philadelphia: Lipponcott Williams & Wilkins, 2001; 433-445.
- Holmgren H, et al: Pain 1990; 40: 43-52.
- Garcia-Larrea L, et al: Brain 2002; 125: 2766-2781.
- Boivie J: Eur J Pain 2003; 7: 339-343.
- Widar M, et al: J Pain Symptom Manage 2004; 27: 215-225.
- Dworkin RH: Am J Med 2009; 122(10 Suppl): S1-2.
- Attal N, et al: Eur J Neurol 2010; 17(9): 1113-1188.
- Leijon G: Pain 1989; 36: 27-36.
- Vranken JH, et al: Pain 2008; 136: 150-157.
- Vestergaard K, et al: Neurology 2001; 56: 184-190.
- Dworkin RH, et al: Pain 2007; 132(3): 237-251.
- Rowbotham MC, et al: N Engl J Med 2003; 348: 1223-1232.
- Attal N, et al: Neurology 2000; 54: 564-574.
- Attal N, et al: Neurology 2002; 58: 554-563.
- Canavero S: Clin Neuropharmacol 2004; 27: 182-186.
- Cruccu G, et al: Eur J Neurol 2007; 14: 952-970.
- Kumar B, et al: Anesthesia and analgesia 2009; 108(5): 1645-1657.
- Khedr EM, et al: J Neurol Neurosurg Psychiatry 2005; 76(6): 833-838.
- Leung A, et al: J Pain 2009; 10(12): 1205-1216.
- Andre-Obadia N, et al: Clin Neurophysiol 2006; 117(7): 1536-1544.
- Fontaine D, Hamani C: Journal of neurosurgery 2009; 110: 251-256.
- Katayama Y, et al: Stereotact Funct Neurosurg 2001; 77: 183-186.
- Owen SL, et al: Pain 2006; 120: 202-206.
- Martin E, et al: Ann Neurol 2009; 66(6): 858-861.
HAUSARZT PRAXIS 2014; 9(5): 20-24