New epidemiological data show that peripheral arterial disease PAVK has assumed a pandemic character worldwide. Although only one-third of patients have symptoms, peripheral arterial disease is an important marker of generalized vascular disease with high morbidity and mortality that is easy to diagnose without much effort. Not making the diagnosis means missing an important preventive approach. The rapid development of new technologies with, in some cases, significantly improved short- and long-term outcomes, also in comparison to open surgical procedures, is increasingly leading to an “endovascular first” strategy” (revised TASC II guidelines).
Peripheral arterial disease (PAVD) affects 202 million people worldwide. That’s almost five times as many as there are HIV-positive patients. National borders, income and standard of living no longer play a role in terms of morbidity. PAVD, as one of the leading causes of morbidity and mortality, has thus assumed a pandemic character [1].
Today, PAVD is still accepted by many as an evil that should not be taken seriously, not least because two-thirds of manifest patients have no symptoms [2]. This fact does not lead patients to the doctor and the doctor does not become aware of it, unless he routinely performs pulse status and ABI measurement during a check-up examination.
Atherosclerosis, the most common cause of PAVD, is anything but a benign condition. Similar to a malignancy, it tends to progress continuously and “metastasize” to the brain, heart, peripheral and visceral arteries. Unfortunately, the current terminology does not help to emphasize this clearly. One speaks of “coronary heart disease”, of “cerebrovascular insufficiency”, of “stroke”, of “stroke”, of “ischaemic insult”, of “claudicatio intermittens”, of “peripheral arterial circulatory disorder”, etc.
In a textbook published in 2002 [3], we have already proposed to stop using this confusing and inaccurate terminology and to speak instead of coronary, cerebral, peripheral, and visceral arterial occlusive disease: A disease with multiple sites. Unfortunately, this did not catch on.
Awareness of the need to aggressively treat cardiovascular risk factors even in the asymptomatic patient with PAVK does not take due precedence-adequate secondary prophylaxis of atherosclerosis is less frequently performed than in the patient with a coronary manifestation, for example. In PAVD patients, only 33% have a beta-blocker, only 29% have an ACE inhibitor, only 31% have a statin, and in known diabetes, only 45% are in the recommended HBA1c level of less than 7% [4]. The risk of critical ischemia or amputation is 1% per year, but the mortality risk is 5 -15%, three to four times higher than in a comparison group of the same age [5].
50% of patients with PAVK have significant coronary involvement and 43% have significant cerebral involvement [6] regardless of whether these patients are symptomatic or not. Not diagnosing PAVD means missing a preventive approach.
The incidence of diabetes mellitus will increase significantly in the coming years. Obesity, caused by lack of exercise as the most common reason, could be easily prevented by preventive measures such as sports and exercise programs [7]. In Germany alone, it is calculated that there will be 1.5 million more diabetics between the ages of 55 and 74 by 2030 [8]. 20% of diabetics over 40 years of age have manifest, usually asymptomatic PAVD, which is an indicator of high amputation and cardiovascular risk. The American Diabetes Association has recommended screening for possible PAVD in all diabetic patients over 50 years of age for the past 10 years [9].
At the beginning of the 19th century, PAVK was still a very rare disease, towards the end it affected mainly the industrialized world and men more often than women. Today, both sexes are equally affected in high-income countries, and in middle- and lower-income countries, women are even more likely to be ill than men.
From 2000 to 2010, PAVK increased by 23.5%. This increase will continue unless appropriate preventive measures are taken. High-income countries lack insight, low-income countries lack money.
Even in medically highly developed countries such as Germany, there is a lack of awareness of the disease, its diagnostic and, above all, therapeutic possibilities among manifestly ill patients.
In an analysis of all patients (n=41882) insured by the largest German health insurance company who were hospitalized for PAVK between 2009 and 2011, their treatment and prognosis were documented (follow-up to 2013) [10]. 4298 underwent amputation, among whom 37% had not received angiography or revascularization within 24 months to amputation, thus were not treated according to guidelines. The lower proportion of angiographies and revascularizations in critical limb ischemia compared with claudication stages is alarming. After index hospitalization, an additional 3527 patients underwent amputation. Such figures make one sit up and take notice, and raise the question as to why such a common, serious disease is not perceived accordingly.
The symptomatic patient
Exercise-induced leg pain significantly reduces quality of life, productivity, and overall health of those affected. Most often manifests itself circulatory disturbance of the legs femoro-popliteal. New interventional catheter techniques and better materials have achieved that there has been a significant improvement in both initial and long-term success, so that today endovascular therapeutic procedures are usually the first line of therapy [11].
The long-term success of percutaneous transluminal angioplasty (PTA) alone is limited by dissection, elastic “recoil,” and restenosis rates as high as 70% at one year.
Nitinol stents, drug-eluting stents (DES), and balloons (DEB) are shown to be clearly superior to PTA in FP lesions.
The longest observation period for a DES is available for the Zilver®-PTX stent (open-cell technique, polymer-free paclitaxel coating, Cook Company). In the prospective, randomized multicenter study of the same name [12], relatively short lesion lengths (mean at 6.5 cm) showed a primary open rate (PP) of 66.4% (overall DES group) compared to 43.4% in the PTA/”bare-metal (BMS)” stent group at five years (p<0.001%). In terms of freedom from target lesion revascularization (TLR), DES was also significantly (p <0.001%) superior to the PTA/BMS group (83.1% vs. 67.6%).
Very good results are also currently emerging for the EluviaTM-PTX stent (biostable polymer-based drug delivery with extended release kinetics, Boston Scientific). The prospective single-arm, multicenter MAJESTIC study [13] found a PP of 96% (49/51) and MAE (major adverse event) rate, defined as any death within 1 month, target extremity amputation, and TLR, of 4% at 12 months, driven exclusively by TLRs. 91% of treated patients (57) showed clinical improvement in Rutherford classification (stage 0 or 1) and ankle-brachial index (ABI, from 0.72 to 1.02). Among them, lesions were slightly longer on average (71 mm) and almost twice as often (65% vs. 37%) severely calcified (46% of them occlusions). Meanwhile, two-year data were presented at the 2016 Cardiovascular and Interventional Radiolocigal Society of Europe (CIRSE) Congress with continued efficacy and safety (TLR 92.5%, MAE 7.5%). Furthermore, there was no stent rupture, nor major amputation.
However, skepticism remains. A limiting factor of DES is the incomplete suppression of neointimal hyperplasia by an inhomogeneous antiproliferative effect between the stent struts and the ends of the stents. In addition, a relatively high concentration of the antiproliferative drug on the “struts” leads to a significantly delayed endothelialization of the stent, which, due to the prothrombogenic metal surface, requires prolonged dual platelet aggregation inhibition. In addition, hardly any other vascular region in our body, due to forces such as compression, torsion, extension, contraction and flexion, poses an enormous challenge to any material used. And with the increasing “endovascular first” strategy (update TASC II guideline [14]), one should try to leave possible bypass landing zones “unencumbered” in the cross-joint segments.
While data from DEBs in the first and second year [15] show themselves to be clearly superior to PTA and at least equivalent to DES [16], robust data in the long-term are scarce. Back in 2015, the five annual results of the first DEB, Thunder study. [17] published, using a small patient population (22 vs. 25 patients in the DEB vs. PTA group), a significantly lower TLR rate (21% vs. 56%, p<0.0005) and a longer interval to reintervention (206 days vs. 607 days, p=0.04) could be elaborated.
The three-year analysis of the prospective, multicenter EU/US, 2:1 randomized, single-blind IN.PACT-SFA trial has now been presented at the VIVA Congress 2016 (Vascular InterVentional Advances). In this study, 330 patients (8% CLI) were treated with the DEB (IN.PACTTM Admiral, n=220) or with the uncoated variant (n=110). The primary efficacy end point (EP) was PP at 12 months, defined as freedom from clinically-indexed TLR and restenosis determined by ultrasound. The primary safety EP is composed of freedom from device- and procedure-related death within 30 days, leg amputation, and clinically-indexed TVR (Vessel). Lesion length averaged 9 cm in both groups. The superiority of DEB over plain PTA already observed at one and two years [1] continues consistently (PP 69.5% vs. 45.1%, ∆ +24.4%), p <0.001; TLR 16.2% vs. 34.0%; p <0.001). The DEB group was also superior to the plain PTA group in the sustainability of clinical improvement, defined as freedom from leg amputation, TVR, and Rutherford classification (68.7% vs. 52.6%, p <0.001). No amputation occurred in either group.
So why not combine the best of both worlds – “Leaving Nothing Behind”?
After initial frustrating data from various uncoated bioresorbable vascular stents (Scaffold, BVS) in the superficial femoral artery (AFS) in 2013 and 2014, the latest generation shows first success (Fig. 1).
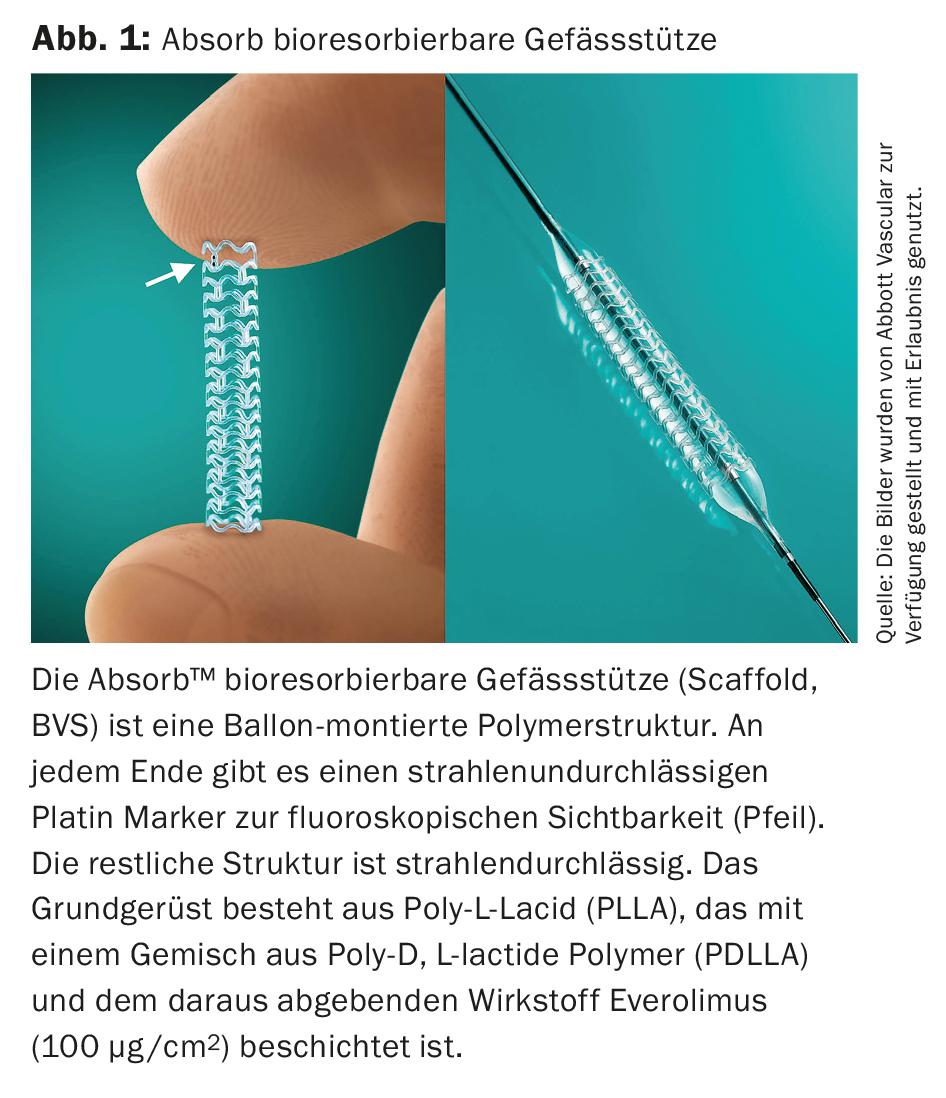
In this context, this BVS system is based on a basic scaffold of poly-L-lacid (PLLA) coated with a mixture of poly-D, L-lactide polymer (PDLLA) and the drug everolimus (100 µg/cm2) delivered from it (ESPRIT™-BVS System, Abbott Vascular, (Fig. 2) . In the prospective, single-arm, multicenter, “first-in human” ESPRIT-I study. [19] 35 patients with symptomatic PAVK and focal (mean approximately 36 mm), poorly calcified lesions (corresponding to TASC A) in the AFS (88.6%) and external iliac artery (11.4%) were studied. After two years, no amputation occurred. The TLR rate was 12.1% at one year, and 16.1% at two years, with a clinically indicated TLR rate of only 9%. The ABI increased from 0.75 before treatment to 0.96 after two years. During this time, 71% of all patients remained largely symptom-free (Rutherford 0); maximum walking distance improved to approximately 450 meters. Limited by the small number of patients, the lack of a direct comparison group, and the relatively short and low calcification lesions, BVS will have to compete in the future in TASC B&C lesions and in comparison with the other DE technologies.

However, it is not sufficient to laboriously open even long-stretch femoro-popliteal occlusions with various instrumentation (Fig. 3) . A greater problem is to keep the reopened vessels open for a longer period of time. Unfortunately, for peripheral interventions, there are no randomized trials to prove which postinterventional therapy is probably best for this, so we have to look to cardiology trials for guidance.
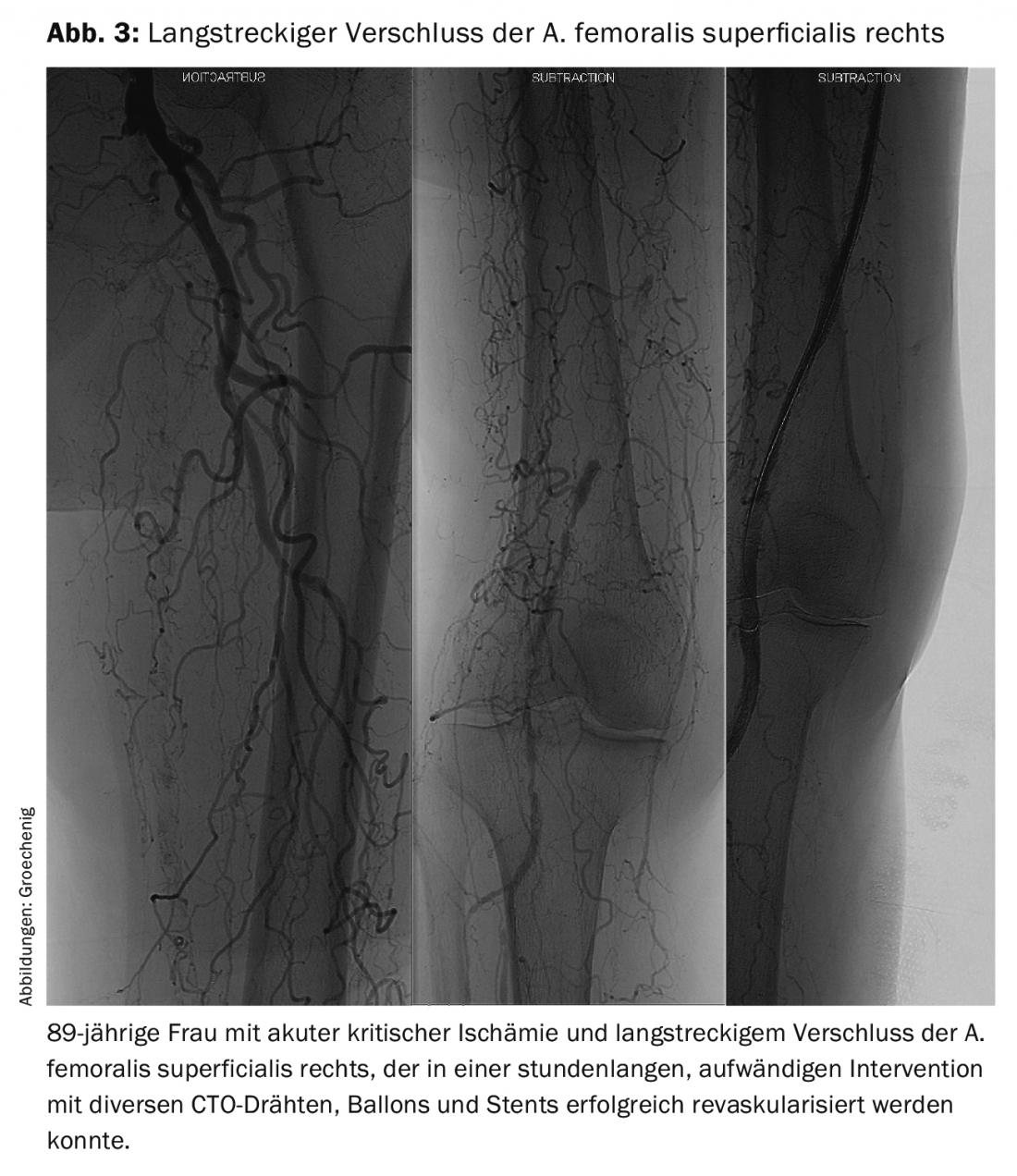
Implanted devices exert a constant growth stimulus on the endothelium, leading to frequent recurrences. However, material fatigue also occurs, especially in the femoro-popliteal section, which is subjected to extreme loads due to torquing, compression, and bending. Such problems are asymptomatic for a long time and then often manifest as acute critical ischemia requiring extensive recanalization (Fig. 4).
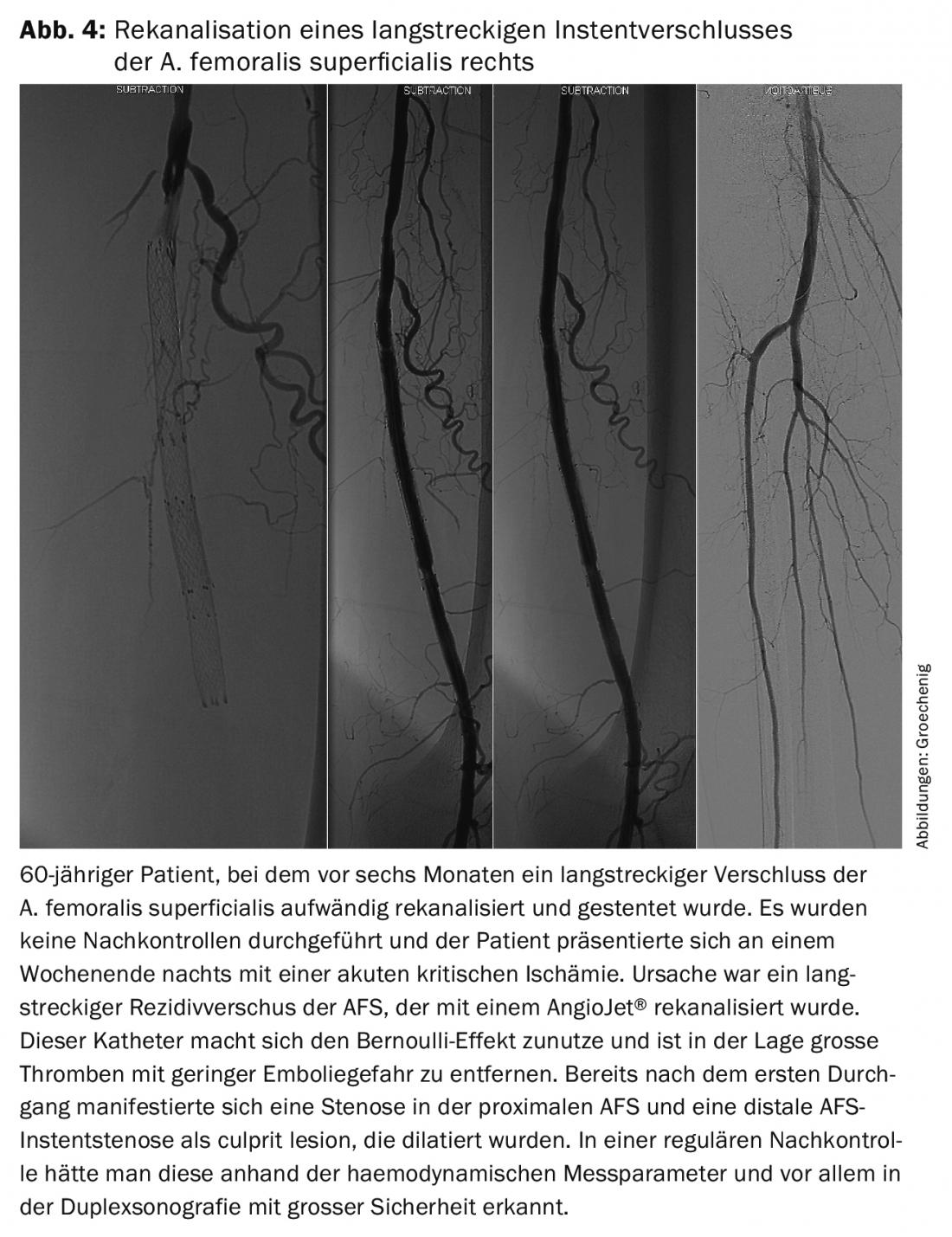
Regular close monitoring is necessary to detect such problems early and to achieve a long secondary, tertiary, quaternary or even more frequent open rate with a minor elective procedure to ensure quality of life and leg preservation for as long as possible.
Summary
In the symptomatic patient, interventional therapeutic procedures are at the forefront with exponential technical development. Adequate and guideline-compliant secondary prophylaxis should be a matter of course. However, this can only delay the course of the disease. While stents have improved the rate of opening of recanalized occlusions, they still have a high recurrence rate due to instent stenosis or material fatigue. In most cases, things then move very quickly and initially asymptomatic patients often present with acute critical ischemia.
Only regular specialist follow-up and early intervention can prevent such disasters. A primary, secondary, tertiary, quaternary, etc. The rate of openness can thus be achieved over many years and thus ensure quality of life and limb preservation. This has long been accepted in bypass surgery, but we still have to work on it in interventional angiology.
Literature:
- Fowkes GR, Rudan D, Ruda I, et al: Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet 2013; 382: 1329 – 1340.
- Diehm C, Darius H, Pittrow D, et al: Prognostic value of a low post-exercise ankle brachial index as assessed by primary care physicians. Atherosclerosis 2011; 214: 364-372.
- Pilger E, Schulte KL, Diehm V, Groechenig R: arterielle Gefässerkrankungen, Standards in Klinik, Diagnostik und Therapie, Thieme Verlag 2002.
- Hirsch AT, Criqui MH, Treat-Jacobson D: The PARTNERS Program. A national survey of peripheral arterial disease prevalence, awareness and ischemic risk. JAMA 2012; 286: 1317-1324.
- Criqui MH, Langer RD, Fronek A, et al: Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992, 326: 381-386.
- Marsico F, Ruggiero D, Parente A, et al: Prevalence and severity of asymptomatic coronary and carotid artery disease in patients with lower limbs arterial disease, Atherosclerosis 2013; 228: 386-389.
- Lindström J, Ilanne-Parikka P, Petonen M, et al: Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study, The Lancet 2006; 368: 1673-1679.
- Brinks R, Tamayo T, Kowall B, et al: Prevalence of type 2 diabetes in Germany in 2040: estimates from an epidemiological model, Euorpean Journal of Epidemiology 2012; 27: 791-797.
- American Diabetes Association: Peripheral arterial disease in people with diabetes: consensus statement, Diabetes Care 2003; 26: 3333-3341.
- Reinecke H, Unrath M, Freisinger E, et al: Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015 Apr 14; 36(15): 932-8. doi: 10.1093/eurheartj/ehv006.
- The TASC Steering Committee. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Endovasc Ther. 2015; 22: 657-671.
- Dake MD, et al: Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the zilver ptx randomized trial. Circulation. 2016; 133(15): 1472-83.
- Müller-Hülsbeck S: Twelve-Month Results From the MAJESTIC Trial of the Eluvia Paclitaxel-Eluting Stent for Treatment of Obstructive Femoropopliteal Disease. J Endovasc Ther. 2016; 23(5): 701-7.
- The TASC Steering Committee. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Endovasc Ther. 2015; 22: 657-671.
- Werner M: Angioplasty with drug coated balloons fort he treatment of infrainguinal peripheral artery disease. Vasa. 2016; 45(5): 365-372.
- Zeller T, et al: Drug-coated balloons vs. drug-eluting stents for treatment of long femoropopliteal lesions. J Endovasc Ther. 2014; 21(3): 359-368).
- Tepe G: Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015; 8: 102-108).
- Laird JR, et al: Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015; 66(21): 2329-38.
- Lammer J, et al: Bioresorbable everolism-eluting vascular scaffold for patients with peripheral artery disease (ESPRIT I). 2-year clinical and imaging results. Am Coll Cardiol Intv. 2016; 9(11): 1178-1187.
CARDIOVASC 2017; 16(1): 18-22