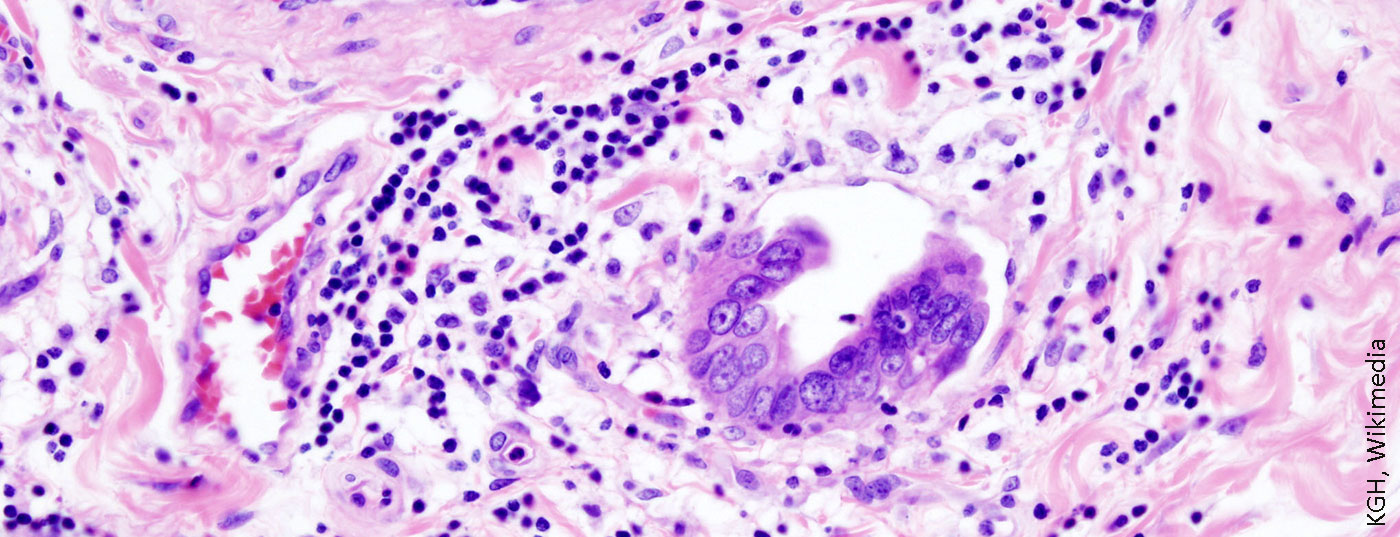
Tumors of the biliary system, such as cholangiocarcinoma or gallbladder carcinoma, are among the rare malignant tumor entities, but they are steadily on the rise. Sometimes combined systemic and locoregional chemotherapy may be considered. In any case, therapy should take place in a specialized center with a multidisciplinary team.
Tumors of the biliary system such as cholangiocarcinoma or gallbladder carcinoma are among the rare malignant tumor entities, but have steadily increased in Europe and Switzerland over the last two decades. This tumor entity spreads along the bile duct system and is often not correctly diagnosed until an advanced stage. That is why the mortality rate of this disease continues to rise. If there is the slightest suspicion, early referral of the patient to a specialized center should be made. Accurate diagnosis of biliary tumors requires a high level of expertise on the part of an interdisciplinary team. An incorrect clarification algorithm may convert a potentially curable treatment option into a palliative situation or delay curative therapy. Treatment of affected patients usually follows a multimodality therapeutic approach including surgical resection, systemic chemotherapy and, in selected cases, radiation therapy. Here, the type of surgery depends on the corresponding tumor localization. In rare cases, liver transplantation is a potential treatment option for patients with perihilar cholangiocarcinoma. This review article reports on the risks and risk factors of this serious disease and explains modern multimodal treatment strategies that have led to a decisive survival advantage for affected patients in the last decade.
How are bile duct tumors classified?
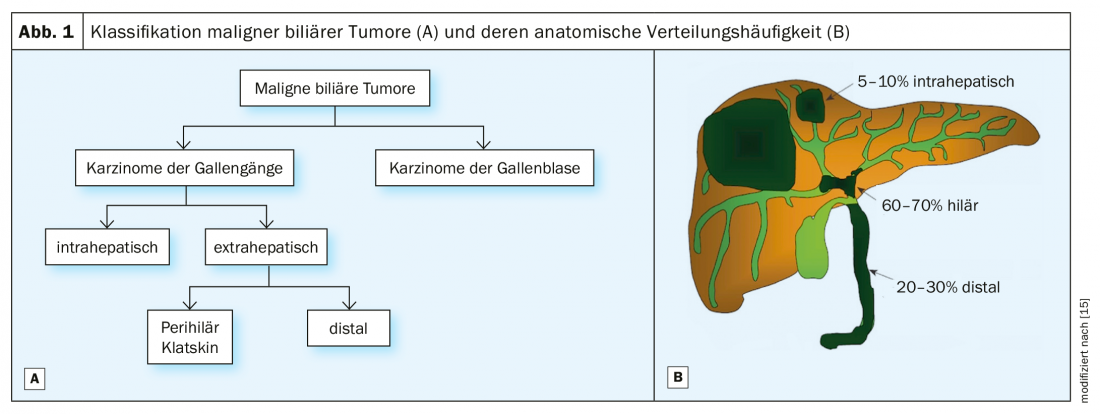
Malignant biliary tumors are divided into carcinomas of the bile ducts (cholangiocarcinomas) and gallbladder, with the former classified into intrahepatic and extrahepatic tumors according to their anatomical location (Fig. 1) . The extrahepatic tumors are further divided into perihilar and distal cholangiocarcinomas. Perihilar tumors, also known as Klatskin tumors, are located immediately at the bifurcation of the extrahepatic biliary system whereas distal cholangiocarcinomas arise more distally in the choledochal duct at the level of the pancreatic head [1]. The surgical treatment strategy is based on this anatomical tumor classification.
How common is cholangiocarcinoma in Switzerland?
Cholangiocarcinoma is a very rare, malignant and heterogeneous tumor disease arising from the epithelial cells of the intra- and extrahepatic bile ducts. This type of cancer represents 3% of all gastrointestinal malignancies [2]. In 2018, 334 new cases were diagnosed in Switzerland. However, in most patients, this cancer is unfortunately detected at a locally advanced stage, which is why the mortality of this tumor disease remains high. Annually, almost half of all patients in Switzerland die after diagnosis (180 deaths per year; corresponding to 1% of all cancer deaths per year) [2].
Who is affected and what are known risk factors?
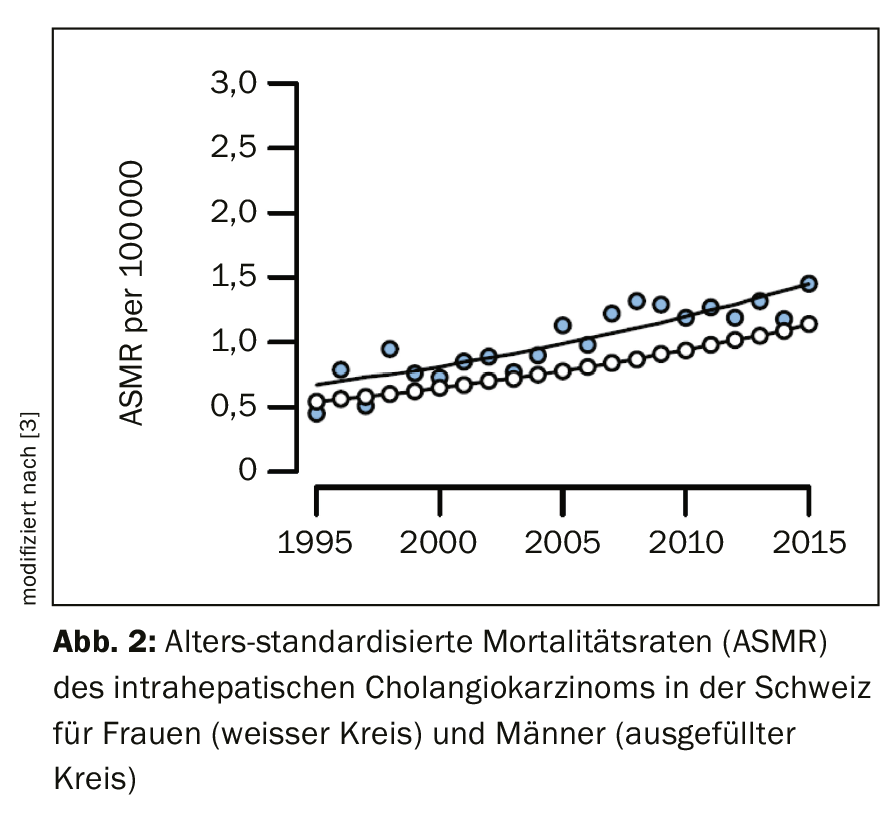
For reasons that remain unclear, the incidence of intrahepatic cholangiocarcinoma has increased over the past two decades in Switzerland and worldwide (Fig. 2), whereas rates of extrahepatic cholangiocarcinoma have declined [3]. In general, the incidence of biliary tract cancer increases with age, reaching its peak between the ages of 50 and 70 [4,5]. Patients diagnosed with primary sclerosing cholangitis (PSC) or known choledochal cysts develop carcinoma nearly two decades earlier on average. In contrast to gallbladder cancer, which affects women at a significantly higher rate, the incidence of cholangiocarcinoma is slightly higher in men [4]. This likely reflects the higher incidence of PSC in men.
A number of risk factors for cholangiocarcinoma are known, although a specific risk factor for many patients cannot be clearly identified [5]. In Europe, the main risk factors are primary sclerosing cholangitis (PSC) and choledochal cysts. There is a clear association between chronic intrahepatic hepatolithiasis leading to recurrent pyogenic cholangitis and cholangiocarcinoma. Chronic liver disease such as cirrhosis or hepatitis B and C are known risk factors, especially for intrahepatic cholangiocarcinoma [5,6]. Furthermore, at least four genetic conditions such as Lynch syndrome, BRCA-associated protein-1 (BAP1) tumor predisposition syndrome, cystic fibrosis, and biliary papillomatosis appear to increase the risk of cholangiocarcinoma [6].
What modern clarifications are necessary?
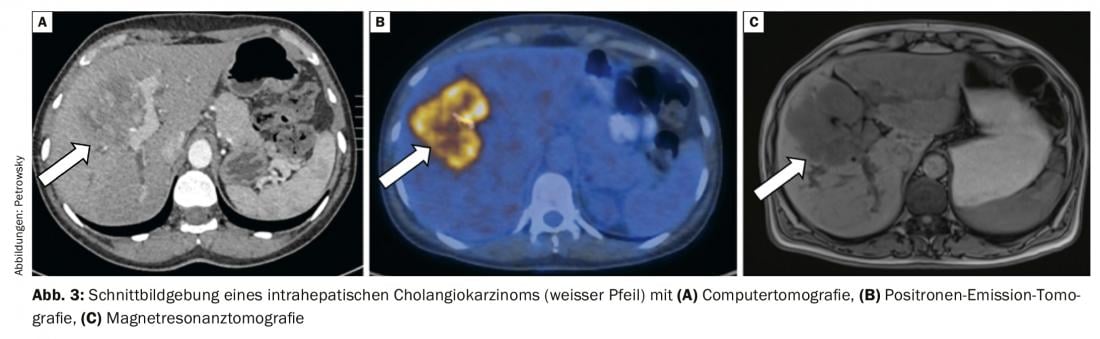
The diagnosis of cholangiocarcinoma usually follows complex work-up algorithms and often requires an infrastructure with pronounced expertise in endoscopic gastroenterology and interventional radiology. If a patient is suspected of having a malignancy of the bile ducts, the first step is topographical classification. Magnetic resonance cholangiopancreaticography (MRCP) represents the best noninvasive method for spatial anatomical visualization of the tumor and, in particular, the bile ducts [7]. It is now almost equal in sensitivity and specificity to the more invasive diagnostic endoscopic retrograde cholangiography (ERC) and percutaneous transhepatic cholangiography (PTC) [7]. Magnetic resonance imaging with MRCP allows assessment of local resectability and also serves as a “route planner” for any endoscopic or percutaneous drainage that may be required. Computed tomography shows increased sensitivity if tumor infiltration of arteries and veins for evaluation before planned resection (Fig. 3) [7].
In the presence of long-standing bile duct thickening, autoimmune cholangiopathy must also be considered as a differential diagnosis. Here, determination of IgG4 in serum and endosonography (EUS) to evaluate the bile duct are helpful [8]. If retroperitoneal and perihilar lymph nodes are abnormal, EUS should also be performed to determine the dignity of the accessible lymph nodes by fine-needle aspiration.
PET/CT is particularly valuable for further staging, especially for the diagnosis of distant metastases, which are often not always diagnosed with regular imaging such as MRI or CT. PET/CT is part of the standardized “work-up” in our clinic and has a weighty influence on the selection of adequate therapy (Fig. 3) [7,9].
After imaging is performed, all patients without exception are presented and discussed at our interdisciplinary tumor board. In particular, this is essential prior to invasive diagnostics such as ERCP or PTCD, as any manipulation of the bile ducts will complicate the assessment of slice imaging.
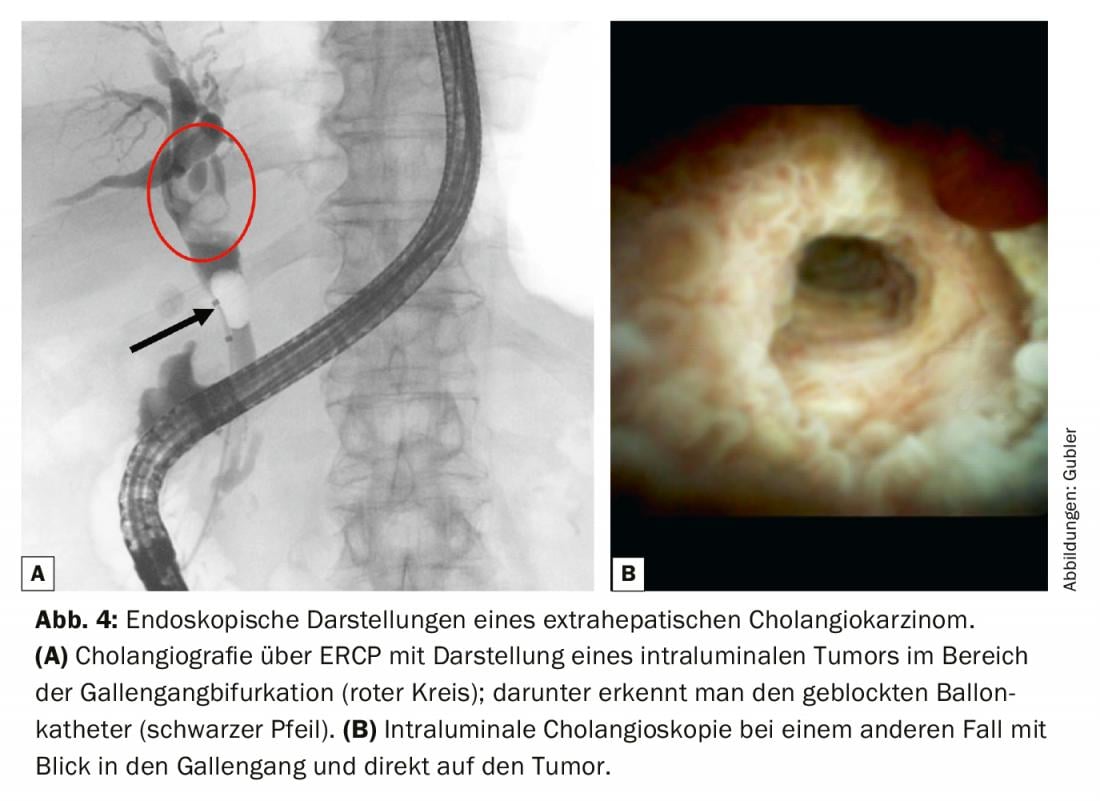
For resectable tumors in the liver or hepatic hilum, a PTCD is preferably inserted preoperatively over the partial liver to be preserved to decompress the biliary system [10]. In the histological diagnosis of perihilar and distal cholangiocarcinomas, ERCP [11] has its firm place. If the bile ducts are filled, drainage must also be performed, otherwise there is a risk of cholangitis with potential liver abscesses. Initially, the most important is to obtain a high-quality cholangiogram with focused acquisition of a brush cytology (Fig. 4). This is usually combined with an intraluminal biopsy to increase sensitivity. If negative tissue diagnosis is not uncommon, peroral cholangioscopy (POCS) with bile duct mapping under visualization can also be performed as a second step. Here, the bile duct bifurcation as well as the right and left bile duct must be visualized in order to use the results of the mapping for surgical planning with estimation of the preoperative resection extent [12]. Staging laparoscopy may be useful to detect small metastases as well as peritoneal carcinomatosis not visible on CT or MRI [13].
What should be considered in surgical resectability?
To evaluate surgical resection, the location of the tumor lesion, including its relationship to vascular and biliary structures, and the volume and quality of the liver parenchyma remaining after tumor resection are critical (Table 1).
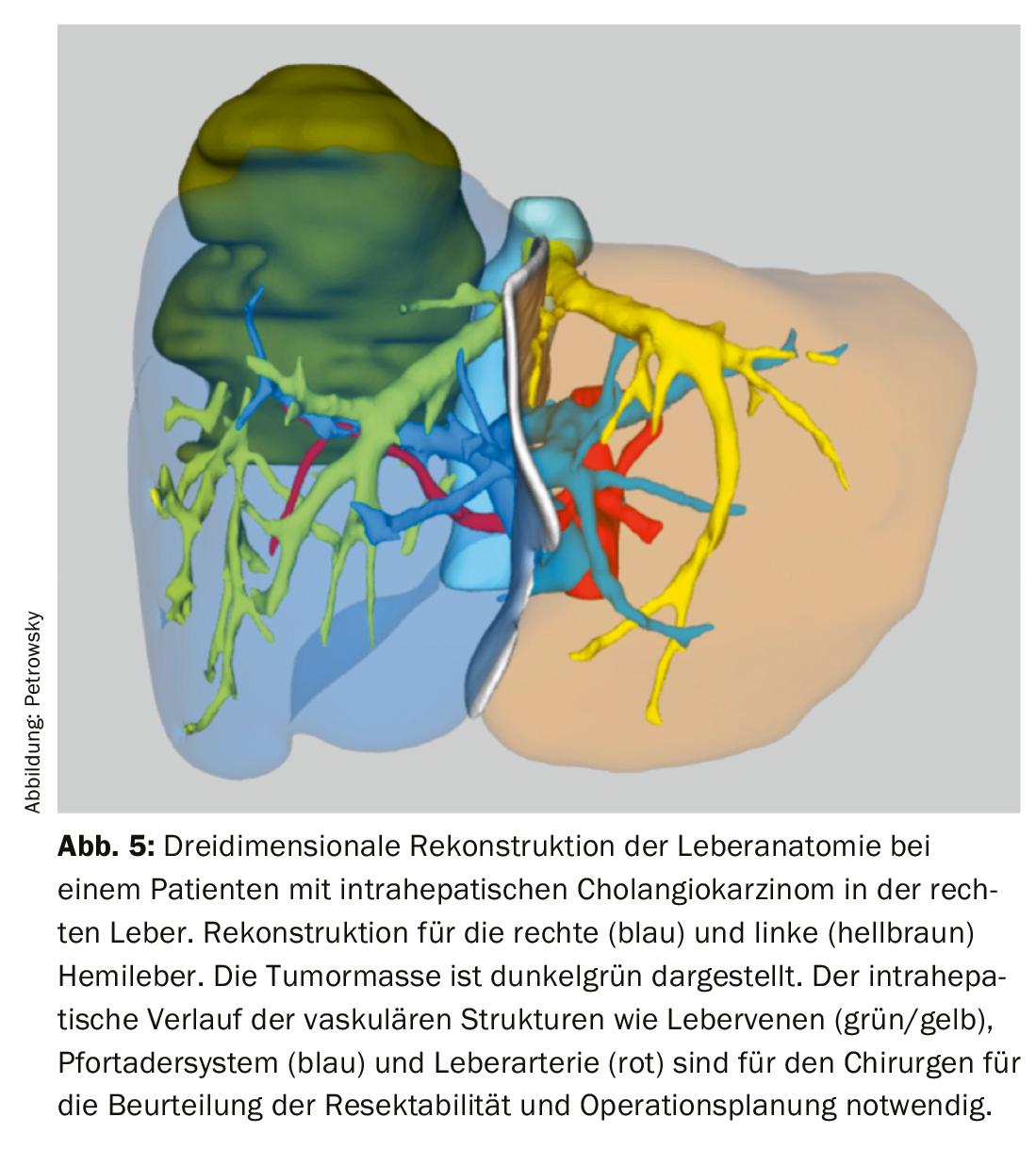
Three-dimensional image reconstructions of vascular and biliary liver anatomy are now available in most centers for accurate assessment of technical resectability and better surgical planning (Fig. 5) . Preoperative histologic tumor detection is always sought prior to surgery, but is not always obtainable in perihilar cholangiocarcinoma. In these cases, a suspicious mass on cross-sectional imaging combined with appropriate clinical data is sufficient to suspect malignancy and indicate resection.
Surgical treatment remains the only curative treatment modality, but only if resection can be performed en bloc with tumor-free resected margins (histologically R0) [14]. Tumor size, satellite lesions, vascular involvement, lymph node metastases, and distant metastases must be considered when assessing resectability. Although tumor size has been controversial in recent years, it was reintroduced into the staging system of the 8th edition of the AJCC [4]. Increasing tumor size is associated with an increased incidence of satellite metastases, lymph node metastases, vascular and poorly differentiated tumors, and thus poorer overall tumor biology [10,15].
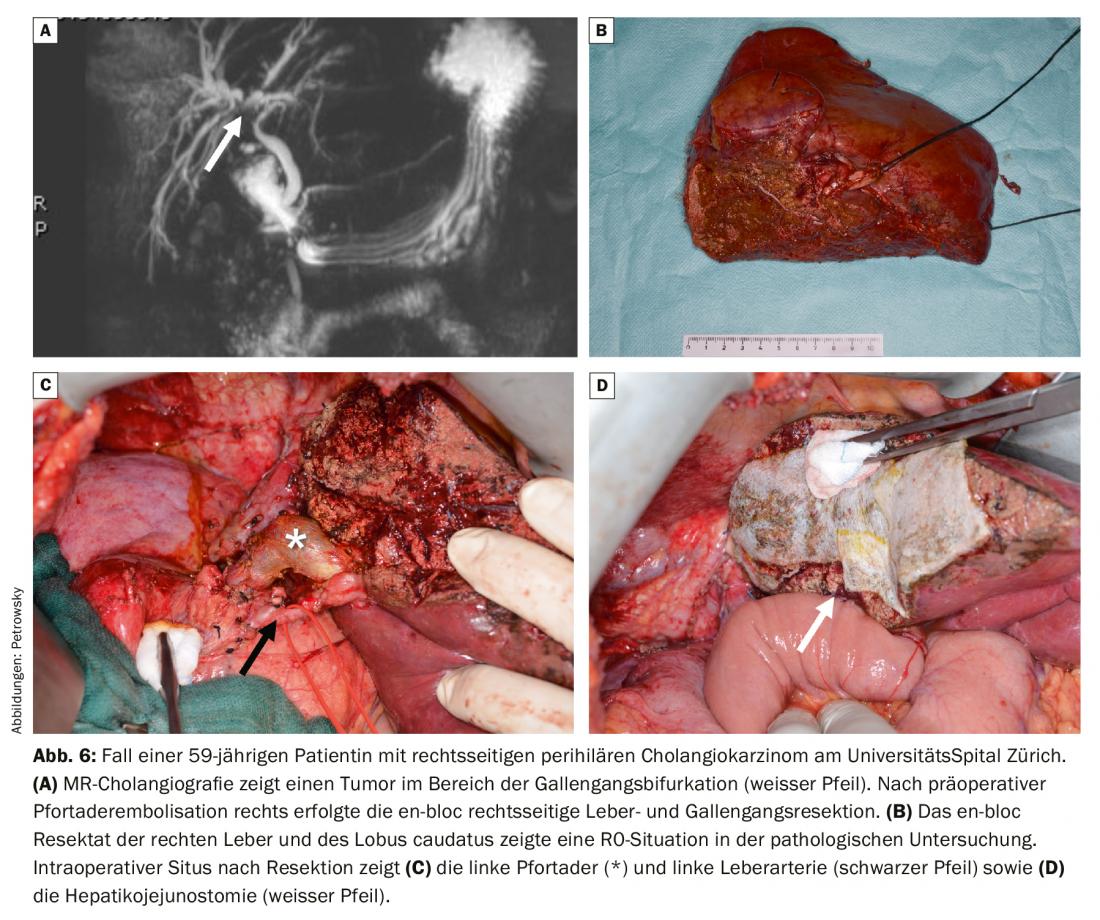
The primary surgical procedure depends primarily on the location of the tumor. Thus, classic pancreatic head resection (Whipple) is suitable for cholangiocarcinomas localized in the distal bile duct. Liver resection of perihilar cholangiocarcinoma is oriented to the side with dominant tumor involvement. Operations of hilar bile duct tumors have a very high complexity and usually require resection of the extrahepatic bile ducts and extended hemihepatectomy including resection of the caudate lobe (Fig. 6) [15]. In cases of tumor infiltration of the portal bifurcation, portal vein resection with primary reconstruction is required [16]. Preoperative volumetry and functional tests of liver function are always performed during these operations. Frequently, these very extensive liver surgical procedures require preoperative portal vein embolization for hypertrophy induction when the remaining liver is too small. In some cases, however, the tumor causes autohypertrophy by tumor occlusion of a portal vein branch, which in this situation no longer requires preoperative portal vein embolization. At our hepatobiliary center, we also drain the remaining side of the liver, usually using a PTCD (percutaneous transhepatic cholangiodrainage), which can then be used as an internal splint and guide structure during surgery. These highly specialized operations should only be performed after careful planning at a center specializing in them.
Intrahepatic cholangiocarcinomas focus on resection of the affected side of the liver. The liver resection procedures required for this are no different from those for other liver tumors such as colorectal liver metastases. Depending on the size and location of the tumor, we offer our patients the full range of surgical techniques from minimally invasive robotic-assisted tumor removal to major complex liver surgery. An important prerequisite is a liver remnant volume that is sufficient in quantity and quality [15]. If, due to tumor size, the extent of resection does not allow sufficient volume of the remaining liver, portal vein embolization for preoperative volume hypertophy or, in selected cases, a two-step liver surgery procedure (ALPPS surgery) may be performed [17]. In the ALPPS procedure, a portal vein branch of the tumor-bearing half of the liver is occluded in an initial operation and the liver parenchyma between the liver halves is cut. This triggers a rapid and substantial growth spurt of that side of the liver with remaining open portal vein. After 1-2 weeks, the tumor can then be easily removed during a second operation, leaving enough liver tissue behind. This strategy achieves R0 resection rates greater than 85%, but should be reserved for cases with unilateral tumor burden of the liver and adequate growth of the tumor-free half of the liver after portal vein embolization [17].
For initially unresectable intrahepatic cholangiocarcinomas, combined systemic and intra-arterial conversion chemotherapy may be used. In this procedure, a pump is used to continuously apply the high-dose chemotherapy directly into the gastroduodenal artery. Intra-arterial therapy can achieve significantly higher drug levels in the liver than systemically. The response rate of this intensified locoregional chemotherapy mostly with floxuridine (FUDR) is 60% and significantly prolongs patient survival even in palliative situations [18]. At our center, we offer this therapy for patients with palliative and potentially curative situations in intrahepatic cholangiocarcinoma. We usually implant the pump subcutaneously in the area of the right lower abdomen and insert the pump catheter minimally invasively using the da Vinci robotic system (Fig. 7). This minimally invasive surgical procedure is very gentle for the patients and ensures a fast postoperative recovery and thus a quick start of chemotherapy after pump implantation.
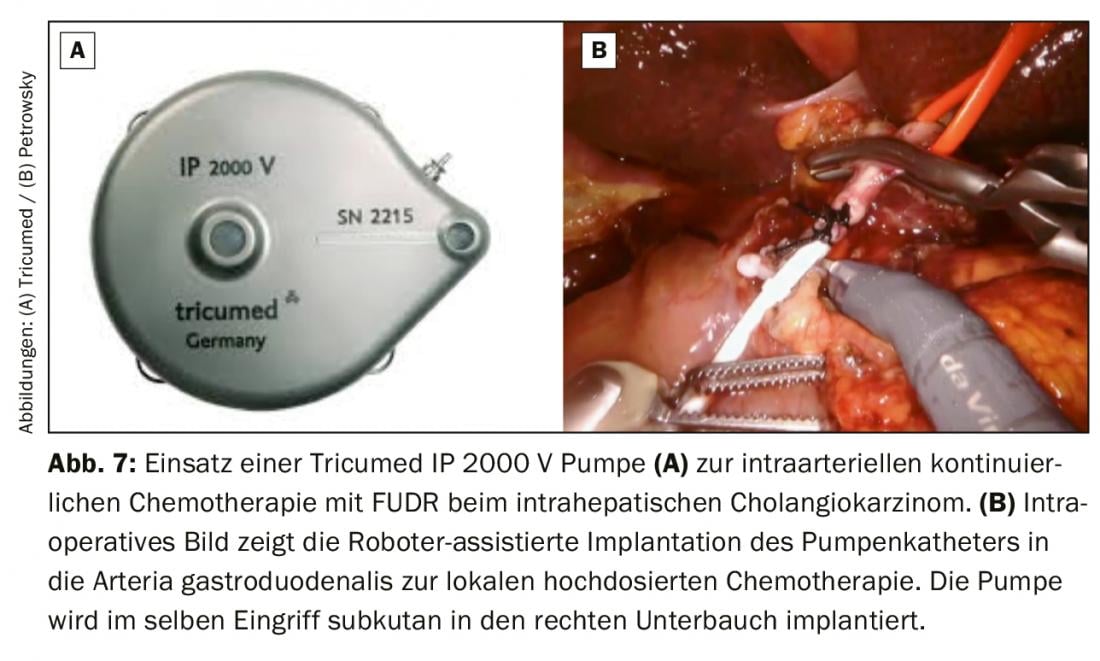
Is liver transplantation a treatment option?
Although liver transplantation technically offers the best possibility of R0 resection, on the other hand, postoperative immunosuppression poses a high risk of tumor recurrence. To date, established criteria for liver transplantation exist only in perihilar cholangiocarcinoma. These are defined by a tumor with a maximum size of 3 cm without evidence of locoregional lymph node and distant metastases. Patients with or without PSC who meet these criteria and are not primarily resectable should be evaluated for liver transplantation with prior neoadjuvant radiochemotherapy. In this context, high survival rates of 60-70% can be achieved 5-years after liver transplantation [19,20]. The indication criteria for liver transplantation for intrahepatic cholangiocarcinoma are less established, although some recent studies report good survival data after transplantation in selected patients [21,22].
Chemotherapy and immunotherapy – What is established and what is new?
In advanced or metastatic disease, systemic chemotherapy remains the standard of care for adenocarcinoma of the biliary tract. To date, no distinction has been made between the individual anatomical subgroups for chemotherapy. The standard of care in first-line therapy is the combination of gemcitabine and cisplatin based on data from the ABC02 trial [23], which demonstrated the superiority of this protocol over gemcitabine mono. For elderly patients and patients with reduced performance status, milder protocols are more appropriate, most likely gemcitabine monotherapy.
Studies on second-line therapy are few to date. Only the ABC06 trial24 demonstrated a survival benefit for patients treated with second-line FOLFOX versus active observation. Alternative second-line treatment protocols that have not been validated by larger randomized trials but are commonly used in practice are FOLFIRI and 5-FU/capecitabine.
However, systemic chemotherapy is used not only for non-resectable or metastatic disease, but also for follow-up after surgery. Here, the BILCAP trial demonstrated a small but significant survival benefit for post-treatment capecitabine [25]. The results of the ACTICCA1 trial comparing capecitabine mono with the activated gemcitabine/cisplatin combination in post-resection treatment are eagerly awaited. Other trial approaches are investigating the role of neoadjuvant therapy, including in incisional gallbladder carcinoma (AIO-HEP-0118, GAIN trial).
Treatment response is currently assessed primarily by cross-sectional imaging. Biochemical progression parameters are mainly the established tumor markers CEA and CA19-9. Extremely exciting and of rapidly increasing clinical importance are the molecular subgroups of biliary tumors. Depending on the primary localization, a whole range of different oncogenic drivers are found in varying frequencies in cholangiocarcinomas. New molecularly targeted substances are available against many of these drivers, some of which are already well advanced in clinical development and are expected to be approved soon. Primary among these are fibroblast growth factor receptor (FGFR) inhibitors, which show excellent efficacy in cholangiocellular carcinomas with oncogenic FGFR alteration (mainly FGFR2 fusions, but also high amplifications) (e.g., pemigatinib or TAS-120). Up to 20% of intrahepatic cholangiocarcinomas show such FGFR alterations, whereas they are rare in extrahepatic tumor locations. IDH1 and IDH2 mutations represent another therapeutic target. These are also common in intrahepatic cholangiocarcinomas and initial randomized trials demonstrate efficacy [26]. Other molecular targets include BRAF-V600E, class II and III BRAF alterations, and HER2. A smaller group of cholangiocarcinomas show microsatellite instability (MSI-H) and respond appropriately well to immune checkpoint blockade [27]. Combinations of molecularly targeted therapy and immunotherapies are currently being investigated in trials (e.g., pembrolizumab plus lenvatinib).
When is stereotactic radiation an option?
Although systemic therapy has been the mainstay in the treatment of locally advanced non-resectable bile duct carcinoma, radiotherapy of local findings has its place in local tumor control and thus in preventing or reducing symptoms or complications. New technical developments such as stereotactic irradiation allow coverage of the tumor area (Fig. 8 ) in a few sessions (usually 5 to 8), with a low side effect profile and without interference or delay of systemic therapy [28–30].
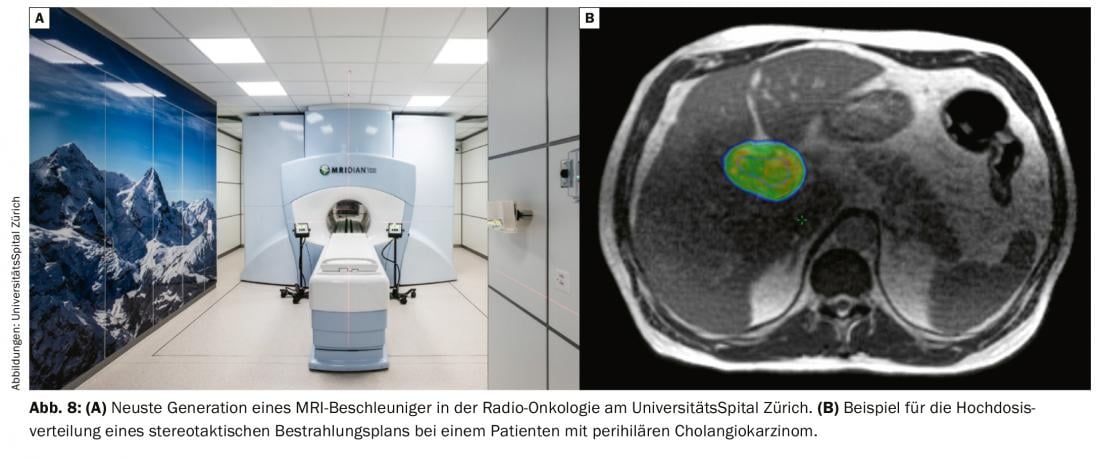
The latest development in this field, so-called MRI-guided irradiation, is so far only available in Switzerland at the University Hospital Zurich in the form of a hybrid MR accelerator (Fig. 7). This technology allows optimal tumor detection with better risk organ sparing through improved soft tissue contrast, real-time imaging during irradiation, and the ability to optimize the plan to the daily anatomy of adjacent organs.
In a large retrospective study, an absolute survival benefit was also observed with the addition of radiotherapy to system therapy [28]. Therefore, in patients with non-resectable cholangiocarcinoma who do not show metastasis in the further course of system therapy, additional local radiotherapy should always be discussed on an interdisciplinary basis.
In which structures is the treatment of cholangiocarcinoma integrated at the University Hospital?
Surgical treatment of cholangiocarcinoma, either by liver or pancreas resection, is part of Highly Specialized Medicine (HSM) in Switzerland. At the University Hospital, the treatment of cholangiocarcinomas is carried out by highly qualified specialists in cooperation with all medical departments. These include liver and pancreatic surgery, medical oncology, endoscopic gastroenterology, interventional radiology and radiation therapy. The University Hospital Zurich offers the latest and most effective treatment options for these tumors and is a certified center for liver cancer, which includes cholangiocarcinomas. The treatments of all patients diagnosed with cholangiocarcinoma are embedded in the structures of the Liver Tumor Center at the Comprehensive Cancer Center Zurich (CCCZ) and the Swiss Center for Liver, Pancreas and Biliary Tract Diseases (Swiss HPB Center). Patients are discussed weekly at an interdisciplinary tumor board focused on liver, pancreas and bile duct tumors. Not only the necessary further diagnostics as well as possible treatment strategy at initial diagnosis are determined, but also the post-therapeutic results and necessary control intervals or secondary therapies are discussed. In addition, our patients have the opportunity to participate in scientific studies conducted at the University Hospital and can additionally benefit from the innovations.
Take-Home Messages
- Intrahepatic cholangiocarcinomas show a worldwide increase while a decreasing trend is observed in extrahepatic cholangiocarcinomas.
- If a bile duct tumor is suspected, referral of the patient to a specialized center should be made as early as possible; involvement of a multidisciplinary team and tumor board is critical.
- Tumor resections of cholangiocarcinomas are part of highly specialized medicine in Switzerland and often require endoscopic and radiological interventional procedures for preoperative preparation.
- Perihilar cholangiocarcinomas (Klatskin tumors), which are primarily localized at the bifurcation of the bile ducts, can also be cured with liver transplantation in selected cases and using a multidisciplinary therapy concept.
- Combined systemic and locoregional chemotherapy may be considered for intrahepatic cholangiocarcinomas; response rates exceed 60%.
- Molecular pathologic investigation should always be considered, especially in intrahepatic cholangiocarcinoma, to identify oncogenic drivers that allow molecularly targeted therapy.
Literature:
- Malhi H, Gores GJ: Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol 45, 856-867, doi:10.1016/j.jhep.2006.09.001 (2006).
- Switzerland K: Cancer in Switzerland: key figures. (2018).
- Bertuccio P, et al: Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol 71, 104-114, doi:10.1016/j.jhep.2019.03.013 (2019).
- Mazzaferro V, Gorgen A, Roayaie S, et al: Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol 72, 364-377, doi:10.1016/j.jhep.2019.11.020 (2020).
- Dervin D: Edmond: is there such a thing as a sick play? Psychoanal Rev 73, 111-119 (1986).
- Chapman RW: Risk factors for biliary tract carcinogenesis. Ann Oncol 10 Suppl 4, 308-311 (1999).
- Olthof SC, et al: Imaging of cholangiocarcinoma. Visc Med 32, 402-410, doi:10.1159/000453009 (2016).
- Strongin A, Singh H, Eloubeidi MA, Siddiqui AA: Role of endoscopic ultrasonography in the evaluation of extrahepatic cholangiocarcinoma. Endosc Ultrasound 2, 71-76, doi:10.4103/2303-9027.117690 (2013).
- Petrowsky H, et al: Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol 45, 43-50, doi:10.1016/j.jhep.2006.03.009 (2006).
- Rassam F, et al: Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience. Langenbecks Arch Surg 403, 289-307, doi:10.1007/s00423-018-1649-2 (2018).
- Abu-Hamda EM, Baron TH: Endoscopic management of cholangiocarcinoma. Semin Liver Dis 24, 165-175, doi:10.1055/s-2004-828893 (2004).
- Ishida Y, Itoi T, Okabe Y: Can image-enhanced cholangioscopy distinguish benign from malignant lesions in the biliary duct? Best Pract Res Clin Gastroenterol 29, 611-625, doi:10.1016/j.bpg.2015.05.007 (2015).
- Bird N, et al: Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br J Surg 104, 418-425, doi:10.1002/bjs.10399 (2017).
- Matull WR, et al: R0 but not R1/R2 resection is associated with better survival than palliative photodynamic therapy in biliary tract cancer. Liver Int 31, 99-107, doi:10.1111/j.1478-3231.2010.02345.x (2011).
- Petrowsky H, Hong JC: Current surgical management of hilar and intrahepatic cholangiocarcinoma: the role of resection and orthotopic liver transplantation. Transplant Proc 41, 4023-4035, doi:10.1016/j.transproceed.2009.11.001 (2009).
- Hemming AW, Mekeel K, Khanna A, et al: Portal vein resection in management of hilar cholangiocarcinoma. J Am Coll Surg 212, 604-613; discussion 613-606, doi:10.1016/j.jamcollsurg.2010.12.028 (2011).
- Li J, et al: ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-center Study. Ann Surg Oncol, doi:10.1245/s10434-019-08192-z (2020).
- Konstantinidis IT, et al: Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 122, 758-765, doi:10.1002/cncr.29824 (2016).
- Darwish Murad S, et al: Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 143, 88-98 e83; quiz e14, doi:10.1053/j.gastro.2012.04.008 (2012).
- Ethun CG, et al: Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann Surg 267, 797-805, doi:10.1097/SLA.00000000002574 (2018).
- Sapisochin G, et al: Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 64, 1178-1188, doi:10.1002/hep.28744 (2016).
- Lunsford KE, et al: Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 3, 337-348, doi:10.1016/S2468-1253(18)30045-1 (2018).
- Valle J, et al: Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362, 1273-1281, doi:10.1056/NEJMoa0908721 (2010).
- ABC-06 | A randomised phase III, m.-c., open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX)
- Lamarca A, et al: Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat Rev 84, 101936, doi:10.1016/j.ctrv.2019.101936 (2019).
- Abou-Alfa GK, Javle TMM, Kelley RK, et al: A global, phase 3, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. ESMO Congress (2019).
- Le DT, et al: Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409-413, doi:10.1126/science.aan6733 (2017).
- Jackson MW, et al: Treatment Selection and Survival Outcomes With and Without Radiation for Unresectable, Localized Intrahepatic Cholangiocarcinoma. Cancer J 22, 237-242, doi:10.1097/PPO.00000000000213 (2016).
- Gkika E, et al: Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer 17, 781, doi:10.1186/s12885-017-3788-1 (2017).
- Brunner TB, et al: Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol 132, 42-47, doi:10.1016/j.radonc.2018.11.015 (2019).
InFo ONCOLOGY & HEMATOLOGY 2020; 8(1): 9-16.