New data presented at the 15th European Epilepsy Congress show long delays in the diagnosis of Lennox-Gastaut syndrome (LGS) in Europe and a significant burden of disease due to seizure and non-seizure related impairments. New treatment options are therefore needed to improve the long-term prognosis of people with LGS.
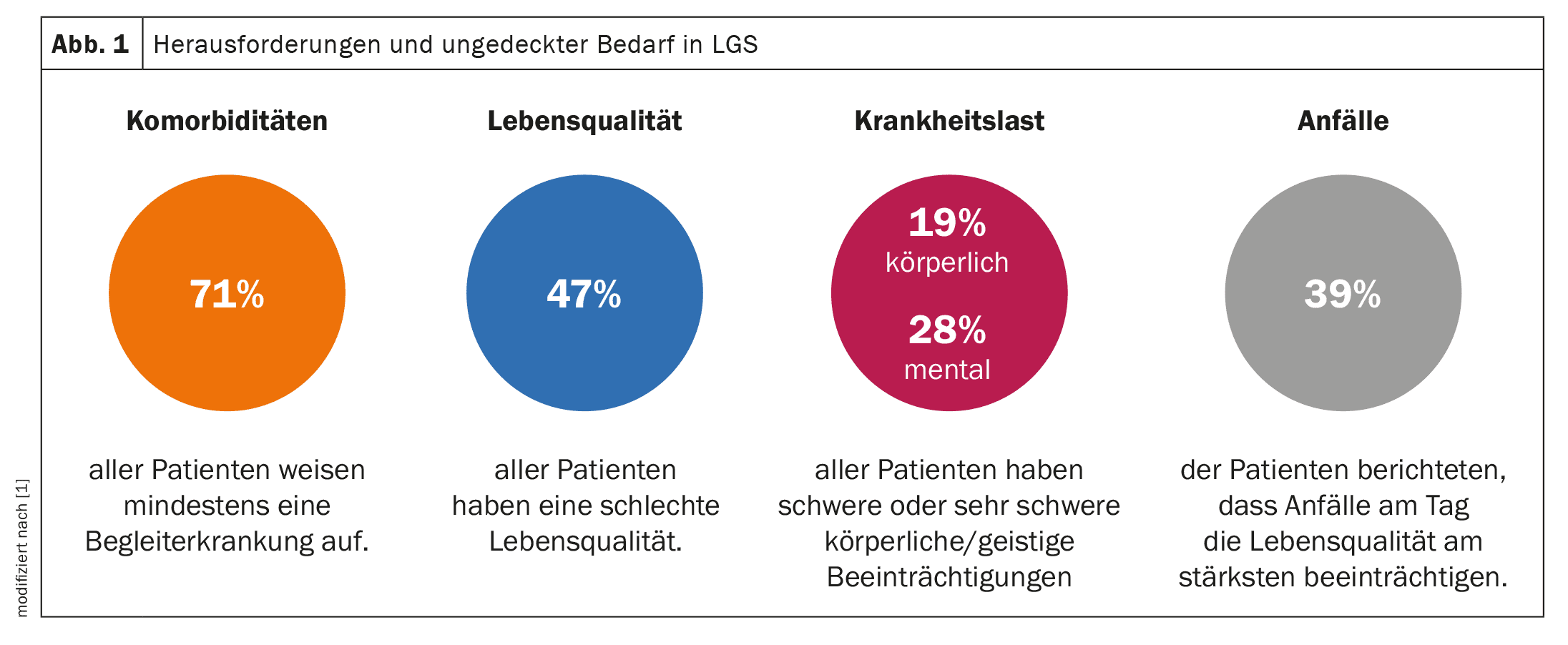
The need for faster and more accurate diagnosis of Lennox-Gastaut syndrome (LGS) has been highlighted in a European real world study based on data from the Adelphi LGS Disease Specific Program (DSP)™. The data from 454 pediatric and adult LGS patients from across Europe shows that it takes an average of 12.3 months for a correct LGS diagnosis to be made after the first seizure occurs at an average age of 4 years. This clearly shows how long patients and their families in Europe have to wait for a correct diagnosis [1]. Despite patients receiving an average of more than three anti-seizure medications per day, challenges remain in the effective treatment of LGS: 71% of patients surveyed had at least one co-existing condition, with psychomotor or cognitive impairment, attention deficit hyperactivity disorder (ADHD), sleep disturbance or insomnia most commonly reported. 19% and 28% of those affected suffered from severe or very severe physical or mental impairments, many of which persisted with increasing age. 47% of patients reported having at least a slightly poorer quality of life and 39% reported that daytime seizures had the greatest impact on their quality of life. These findings underscore the need for new treatments that target both drug-resistant seizures and the non-seizure symptoms of LGS.
LGS affects approximately one million people worldwide [2], but diagnosis remains complex and challenging due to the lack of specific biomarkers for the disease, multiple possible causes and variable symptoms [3]. Although several approved drugs are available for LGS, there is still an unmet need for treatment. These medications provide improvement but generally do not have a sustained long-term effect [4].
Diagnostic support for medical professionals
Details of a new electronic decision support tool for LGS, based on the International League against Epilepsy (ILAE) diagnostic criteria, were also presented. Developed by ten epilepsy experts, the tool, whose digitization was funded by UCB (with no influence on the content), is designed to help physicians assess the likelihood that their patients have LGS. The questionnaire takes into account the mandatory, warning and exclusion features of LGS and provides a result that can be a guide for future treatments [5,6].The prototype is now being tested and validated before it can be used by healthcare professionals in the clinical setting.
Source: UCB
Literature:
- Strzelczyk A, et al: Insights into Lennox-Gastaut syndrome: A European real-world study on patient profiles and unmet needs. Poster presented at: The15th European Epilepsy Congress (EEC); 2024, September 7-11; Rome, Italy.
- www.lgsfoundation.org/wp-content/uploads/2024/05/Updated-MAY-2024.png. Last accessed: August 2024.
- Bourgeois BFD, Douglass LM, Sankar R. Epilepsia. 2014;55(Suppl. 4): 4-9.
- Auvin S. Revue Neurologique. 2020; 176: 444-447.
- Arzimanoglou A, Specchio N, Auvin S, et al: Development of an Electronic Diagnostic Criteria Tool for Lennox-Gastaut Syndrome (LGS). Poster presented at: The15th European Epilepsy Congress (EEC); 2024, September 7-11; Rome, Italy.
- Specchio N, Wirrell EC, Scheffer IE, et al: Epilepsia. 2022; 63(6): 1398-1442.
InFo NEUROLOGY & PSYCHIATRY 2024; 22(5): 37