For almost two years, an effective therapeutic option has been available for patients with non-resectable or metastatic BRAFV600-mutated melanoma with the combination of encorafenib plus binimetinib. An analysis of the COLUMBUS trial presented at this year’s ASCO shows that the beneficial treatment effects are maintained after a four-year period.
In Switzerland, the BRAF/MEK inhibitor combination therapy encorafenib (BRAFTOVI®) plus binimetinib (MEKTOVI®) has been approved since the end of 2019 for adult patients with non-resectable or metastatic melanoma with a BRAFV600 mutation [1,2]. Follow-up analyses of the pivotal COLUMBUS study show that the positive balance in terms of clinical efficacy parameters as well as tolerance remains after a period of four years. In the context of the German Skin Cancer Congress ADO 2020, Prof. Dr. med. Axel Hausschild (Kiel/D) , Prof. Dr. med. Dirk Schadendorf (Essen/D) and PD Dr. med. Sebastian Haferkamp (Regensburg/D) spoke about the current data situation of targeted combination therapy, future therapy strategies in malignant melanoma and “real world” evidence [9,11,16].
COMBO450 combination therapy proves to be permanently effective
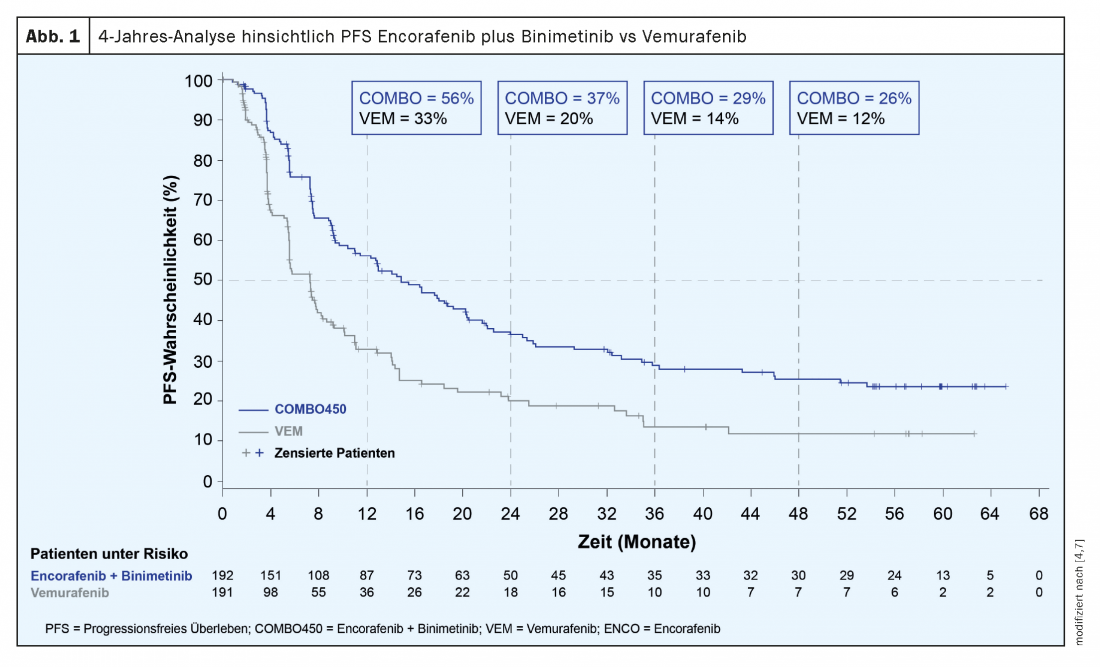
In the three-arm phase III COLUMBUS study, the combination of encorafenib plus binimetinib (COMBO450) was compared to the monotherapies encorafenib and vemurafenib, respectively. Included were 577 patients with newly diagnosed advanced/metastatic BRAFV600-mutated melanoma or progression after first-line immunotherapy [3]. The targeted combination therapy COMBO450 proved significantly superior to monotherapy with vemurafenib (7.3 months) and encorafenib (9.6 months), respectively, with a median PFS of 14.9 months. A follow-up analysis of the COLUMBUS trial presented at this year’s ASCO shows that patients continue to benefit from targeted combination therapy after four years [4]. With a 4-year survival rate of 39% (95% CI: 32-46), the long-term efficacy of the combination of Braftovi® plus Mektovi® was demonstrated. The corresponding figures were 37% (95% CI: 30-44) and 26% (95% CI: 19-32) among encorafenib and vemurafenib monotherapies, respectively. This is similarly confirmed by the rate at PFS after four years of therapy, as this was 26% (95% CI: 19-33) in the combination arm versus 22% in the encorafenib arm (95% CI: 15-29) and 12% in the vemurafenib arm (95% CI: 6-20) without progression (Fig. 1). This indicates a long-lasting efficacy of the recent combination. The overall response rate was also confirmed compared to previous evaluations [3,5,6].
The median follow-up in terms of OS across all arms was 60.6 months. A median OS of 33.6 months was achieved in the combination arm with encorafenib plus binimetinib (95% CI: 24.4-39.2), compared with 23.5 months in the encorafenib arm (95% CI: 19.6-33.6) and 16.9 months in the vemurafenib arm (95% CI: 14.0-24.5). Thus, patients on combination therapy had a 39% reduced risk of death compared with vemurafenib (HR=0.61; 95% CI: 0.48-0.78) [7].
The safety data of the combination therapy were consistent with the known profile. Side effects are generally well managed, potentially limiting events during therapy with encorafenib plus binimetinib such as fever (20%) or phototoxicity (4%) were observed with rather lower frequency. The most common adverse events (≥25%; all grades) during combination therapy were nausea, diarrhea, vomiting, fatigue, arthralgia, CK elevation, headache, and constipation [4].
| TAVIE Skin App (Tremfya®) The TAVIE Skin App digital tool is designed for patients undergoing oral melanoma therapy. In several video courses, those affected learn to better understand their disease and how to deal with potential side effects of the therapy. The reminder function helps patients prepare for their next doctor’s appointment, with only the practitioner being able to view the data at the patient’s request. Presented at the 10th European Post-Chicago Melanoma/Skin Cancer Meeting 2020, the TAVIE Skin App is expected to be available free of charge in German on the AppStore and GooglePlay for all melanoma patients on oral therapy starting this fall. |
Further treatment strategies on the test bench
Another treatment approach is to combine immunotherapy (checkpoint inhibitors) with targeted therapy (BRAF/MEK inhibition) in the hope of achieving a faster response and more long-lasting therapeutic effects [11]. KEYNOTE-022 is a randomized phase II trial comparing triple therapy of dabrafenib, trametinib and pembrolizumab with the combination of dabrafenib plus trametinib in patients with BRAF-mutated advanced melanoma. Although PFS and OS were found to be slightly higher in the triple therapy treatment arm than in the two-drug combination, this was accompanied by significantly increased toxicity of a [11]. So far, it is unclear which patients might benefit from such a triple therapy and whether a sequential or a simultaneous treatment leads to better results.
The LOGIC-2 trial is an international, multicenter, open-label, 2-part phase II study that investigated a sequencing approach: The combination of the BRAF inhibitor LGX818 and the MEK inhibitor MEK162 followed by LGX818 plus MEK162 with different agents such as BKM120 (PI3K inhibitor), BGJ398 (selective inhibitor of FGFR), INC280 (MET inhibitor) or LEE01 (CDK4/6 inhibitor) after progression in patients with non-resectable or metastatic melanoma and BRAF V600 mutation [8]. The conclusion is that the overall efficacy of this triple combination after dual blockade has occurred is relatively low and only certain types of patients benefit from triple combinations [9,10].
Testing high-risk patients for BRAF mutation
BRAF mutation activation occurs in approximately 50% of cutaneous melanomas [12,13]. To date, approximately 300 BRAF mutations have been identified, the most common of which is V600E (valine to glutamic acid; 70-88%) [12,14]. As a basis for personalized therapy, early testing for the presence of a BRAF mutation should be performed in stage II, III and IV high-risk melanoma patients according to the ESMO Clinical Practice Guidelines [15]. Patients with unresectable metastatic melanoma who test positive should receive therapy with a BRAF/MEK combination. BRAF/MEK inhibition inhibits the mitogen-activated protein kinase (MAPK) pathway, which is continuously activated in BRAFV600 mutation.
Literature:
- Technical information BRAFTOVI®, https://compendium.ch, last accessed 16.09.2020
- Technical information MEKTOVI®. https://compendium.ch, last accessed 16.09.2020
- Dummer R, et al: Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19(5): 603-615.
- “COLUMBUS trial update: patients reap long-lasting benefits,” Pierre Fabre, September 08, 2020.
- Dummer R et al. Lancet Oncol 2018; 19(10): 1315-1327.
- Ascierto PA et al: Eur J Cancer 2020; 126: 33-44.
- Gogas H et al. ASCO2020: Abstract #10012 and Poster #361.
- Dummer R: A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAFV600-mutated melanoma (LOGIC2). https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.10022
- Hauschild A: Update COLUMBUS. Prof. Axel Hauschild, MD, 20th German Skin Cancer Congress, ADO Annual Meeting, September 11, 2020.
- Dummer R et al: ASCO 2020: Abstract #10022 and Poster # 371.
- Schadendorf D: Update COLUMBUS. Prof. Dirk Schadendorf, MD, 20th German Skin Cancer Congress, ADO Annual Meeting, September 11, 2020.
- Tanda ET, et al: Current State of Target Treatment in BRAF Mutated Melanoma. Front. Mol. Biosci2020, https://doi.org/10.3389/fmolb.2020.00154
- Sanchez-Vega F, et al: Oncogenic Signaling pathways in the cancer genome atlas. Cell 2018; 173: 321-337.e10.
- Menzies A, et al: Distinguishing Clinicopathologic Features of Patients with V600E and V600K BRAF-Mutant Metastatic Melanoma. Clin. Cancer Res 2012; 18, 3242-3249.
- Michielin O, et al. and Esmo Guidelines Committee. Ann. Oncol. 30, 1884-1901.
- Haferkamp S: Update COLUMBUS. PD Dr. Sebastian Haferkamp, 20th German Skin Cancer Congress, ADO Annual Meeting, September 11, 2020.
DERMATOLOGY PRACTICE 2020; 30(5): 52-53