A promising approach for the therapy of multiple sclerosis lies in antigen-specific treatment. They are intended to specifically inhibit the autoreactive immune response to avoid the risks of global immunosuppression.
Immune-mediated diseases, such as autoimmune diseases, have so far been treated by non-specific and global immunosuppressive therapies. This often results in side effects and increased risks of complications. Multiple sclerosis (MS) is a paradigmatic autoimmune disease of the central nervous system. The development of new therapies for MS has been very successful over the past decade, and we now have a variety of new and highly effective drugs available. However, all these immunotherapies have a non-specific effect and lead to a sometimes deeper immunosuppression, which is associated with the known side effects and sometimes high risks. Another consequence is a limitation in many areas of life such as family planning, vaccinations and travel.
The development of antigen-specific therapies that treat with high selectivity only those portions of the immune system responsible for autoreactive inflammation would have the potential to treat the disease close to its cause and avoid the risks of global immunosuppression [1]. Successful implementation of this therapeutic strategy would be a major milestone in medicine and a further step towards precision medicine.
After initial studies with antigen-specific therapies mostly failed to achieve the hoped-for success, there has been important progress in this field in recent years, so that a successful implementation of this promising therapeutic concept is within reach. These include innovative therapeutic strategies, which are already in early clinical development, and the identification of new target antigens of the autoreactive immune response of MS patients, which will enable entirely new approaches for antigen-specific therapies [2,3].
An essential prerequisite for the development of antigen-specific therapies is a good understanding of the pathogenesis of the disease and the target antigens of the autoreactive immune response. The cause of MS is not known, but both environmental and genetic factors are thought to contribute to the onset and progression of the disease. By far the most important genetic factor is a specific human leukocyte antigen (HLA), a surface molecule responsible for the recognition of antigens by T cells and thus an important factor in profiling individual immune responses. On average, 50% of MS patients carry the major risk allele HLA DRB1*1501, with the remainder divided between several other HLA alleles. The individual HLA type should therefore be considered in the future when testing antigen-specific therapies in MS patients. In addition, there are a variety of findings suggesting an immune-mediated immune response in MS directed against specific autoantigens [4].
The antigen spectrum in MS
A number of different target antigens have been described in multiple sclerosis. Based on the pathophysiology with demyelination in the CNS in the foreground, mainly proteins of the myelin layer of neurons in the brain and spinal cord were investigated. The most important proteins in this context are myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte gylocoprotein (MOG). In various animal models in mice, rats, and monkeys, an induced immune response against one of these proteins can cause an inflammatory demyelinating disease that resembles MS in many characteristics. This immune response is mostly directed against a few peptide sequences from these proteins, which are described as immunodominant. Interestingly, these immunodominant peptides in animals largely overlap with the immune reactivities found in MS patients [4]. In a study of MS patients, seven myelin peptides (MOG1-20, MOG35-5, MBP13-32; MBP83-99, MBP111-129, MBP146-170, PLP139-154) were identified from a larger number of immunodominant peptides of the myelin proteins MBP, PLP, MOG, and cyclic nucleotide phosphodiesterase (CNP), for which reactivity in MS patients differed from controls [5]. This set of peptides now serves as the basis for antigen-specific therapies in several approaches.
In addition to myelin proteins, other proteins may play important roles as target antigens of the autoreactive immune response in the CNS, and most recently, two new candidates have been identified as important target antigens in MS patients. In contrast to previous studies, which were essentially based on pre-existing pathophysiological concepts and translated from animal models to humans, these recent studies have taken MS patients as the starting point and investigated which peptide sequences are recognized by T cells isolated directly from inflammatory brain tissue of MS patients [3]. In this context, the TSTA3 protein, a GDP L-fucose synthase, was discovered to be a target antigen of the autoreactive immune response [3]. An interesting and most likely pathophysiologically relevant aspect is that the protein is also expressed by certain intestinal bacteria (Akkermansia), which are frequently found in MS patients. These bacteria could contribute to the immune activation, which then leads to or maintains the inflammation in the brain. Another important target antigen is the protein RAS Guanyl Releasing Protein 2 (RASGRP2), which is expressed in B cells in both peripheral blood and brain. T cells from MS patients that are activated in peripheral blood, tend to increase autoproliferation, and preferentially migrate to the brain show increased reactivity against RASGRP2 protein and can be detected in lesions from MS patients [2].
It is not likely that the pathogenic immune response of an MS patient, or in MS in general, is directed against only a single antigen, but may involve multiple antigens. Moreover, it has been shown in animal models that this autoreactivity can diversify and extend to different antigens during the course of the disease. In humans, there is little data on this to date, but it is important because in most cases the onset of disease is not clearly established and therefore the cascade of diversification, and thus the full spectrum of antigens in the individual patient, is not known.
Mechanisms of immune tolerance
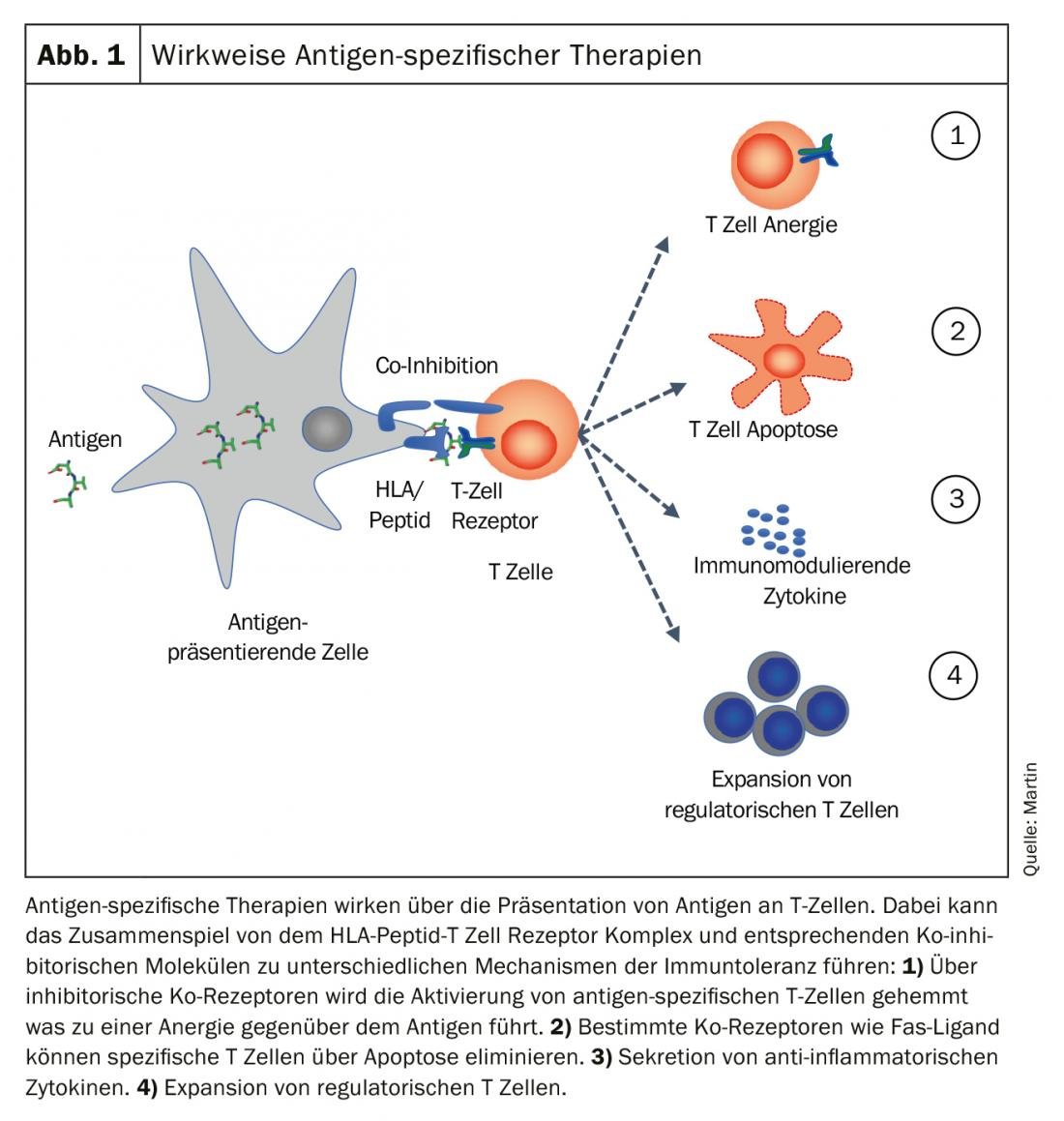
The goal of antigen-specific therapies is to “re-establish” immunological tolerance to the specific antigen. Different mechanisms, alone or in combination, can contribute to the establishment and maintenance of immune tolerance. The main four mechanisms are shown in Figure 1.
- The antigen-presenting cell can prevent the activation of antigen-specific T cells via inhibitory co-receptors and generate anergy toward the antigen.
- Co-receptors such as Fas ligand can induce apoptosis in specific T cells and eliminate them.
- A tolerogenic milieu is created via secretion of anti-inflammatory cytokines and the expansion of regulatory T cells is induced.
Antigen-specific therapies in clinical trials
Different strategies using antigen-specific therapies have been tested in clinical trials in MS patients to date. Most of them focused either on single myelin proteins or few peptides as target antigens for tolerance induction. Only a few approaches have used multiple peptides from different myelin proteins. The routes of administration tested were oral, transdermal, epi- and subcutaneous, and intravenous. After negative study results on oral administration of myelin or intravenous application of MBP peptides, T-cell or T-cell receptor vaccination, some therapeutic approaches have recently shown positive study results [1]. A phase II study investigated the transdermal administration of three myelin peptides MOG35-55, MBP85-99, and PLP139-151 applied via a patch on the upper arm [6]. The study in 30 relapsing MS patients met the study endpoint of a reduction in contrast-enhancing foci on cerebral MRI. The authors were also able to show a reduction in peptide-specific T cells at a mechanistic level and describe the importance of dendritic cells in draining lymph nodes [7]. Another therapeutic approach uses four MBP peptides, which are applied parenterally. A phase II study in 37 relapsing patients who received biweekly intradermal injections of MBP peptides demonstrated a significant reduction in contrast-enhancing lesions on cerebral MRI [8]. The effect was dose-dependent and persisted for the duration of treatment. Intramuscular injection of a DNA leading to the expression of MBP protein in muscle cells was tested in 267 patients. The study did not meet the primary endpoint but demonstrated a reduction in new MR lesions [9]. The procedure is currently being pursued in the treatment of type I diabetes. A small phase Ib clinical trial with tolerogenic dendritic cells has confirmed the safety and tolerability of the procedure and shown evidence for an effect on autoreactive immune cells [10]. In the procedure, autologous monocytes were obtained by leukapheresis and tolerogenic dendritic cells (tolDCs) loaded with myelin peptides were grown in vitro. The tolDCs were injected intravenously in eight MS patients.
In addition to the specific strategy and route of application, the selection and presumably the number of antigens targeted by the therapy is important for the success of the procedure. One therapeutic strategy we have pursued in recent years is intravenous injection of blood cells (leukocytes or erythrocytes) that have been coupled ex vivo at the cell surface with peptides. The approach allows the use of a larger number of peptides to inhibit the antigen-specific immune response simultaneously against multiple antigens. In animal models, this was very effective in preventing both disease onset (prophylactic) and progression (therapeutic), and the therapy was successful in various disease models of autoimmune disease, allergy, and transplantation. In addition, antigen-coupled cells are the only method that has been shown to prevent diversification of the immune response.
An initial phase Ib study in nine MS patients evaluated the safety and tolerability of autologous peripheral blood mononuclear cells coupled ex vivo with seven myelin peptides from MOG (MOG1-20, MOG35-55), MBP (MBP1-32, MBP83-99, MBP111-129, MBP146-170) and PLP (PLP139-154) [11]. Overall, the therapy was very well tolerated and the course of MS remained stable. Concomitant immunologic studies also found no evidence of inflammatory activity after therapy, and a reduction in peptide-specific T-cell reactivities was demonstrated in patients receiving the highest dose. These results were recently confirmed also with autologous erythrocytes as tolerogenic carrier cells and evidence for a beneficial effect at cellular and humoral levels was found [Lutterotti et al. ECTRIMS Congress, Stockholm, 2019]. An extension of this Phase Ib study is ongoing. In the future, additional peptides from new antigens may be added to the procedure, making it the most comprehensive set of autoantigens yet used in an antigen-specific therapy procedure.
Current and future challenges
In the era of precision medicine, antigen-specific therapies are again becoming the focus of new therapeutic developments. This trend is also supported by a better understanding of the autoimmune genesis of the disease and the identification of new target antigens.
New technologies, such as nanoparticle- and cell-based approaches, are already in early preclinical development and will expand the range of therapeutic options in the future. A major challenge for the evaluation and comparability of new therapeutic approaches is the biological demonstration of induced immune tolerance. To date, there are no standardized biomedical methods to confirm a reduction in the autoreactive immune response at the cellular or humoral level after therapy. The development of assays to identify and quantify antigen-specific immune responses following appropriate therapy are an important aspect of clinical development and should also be used to select and stratify patients in clinical trials of antigen-specific therapies. Ideally, one would select patients based on individual immunoreactivity, measure the reduction in specific immune response after treatment, and correlate it with clinical response. In addition, it is a relevant safety aspect to exclude activation of the immune system by therapy, as this could lead to increased disease activity [12].
Antigen-specific therapies will improve the therapeutic armamentarium for the treatment of MS and should ideally be used early in the disease to prevent further propagation of autoreactive immune responses. The good tolerability and high safety profile of the therapeutic approach are an advantage over current non-specific therapies, especially in the early phase of the disease. In the future, especially in patients with high disease activity, the combination of antigen-specific approaches with current standard therapies should also be investigated, with the aim of achieving rapid control of inflammation and treating only with antigen-specific therapy in the further course. This can avoid the long-term risks of current therapies.
A major question will be whether long-term immune tolerance, i.e., complete elimination of autoreactivity even after discontinuation of therapy, can be achieved or whether permanent antigen-specific therapy must be administered to control the disease. In any case, antigen-specific therapies would revolutionize the treatment of MS and other autoimmune diseases and represent a major advance in medicine.
Take-Home Messages
- Antigen-specific therapies are a promising therapeutic concept for the treatment of multiple sclerosis.
- The goal of antigen-specific therapies is to specifically inhibit the autoreactive immune response and avoid the risks of global immunosuppression.
- The identification of novel target antigens in MS are an important basis for the development of antigen-specific therapies.
- Different therapeutic approaches are already in early phases of clinical development and are showing initial success with regard to their efficacy.
Literature:
- Lutterotti A, Martin R: Antigen-specific tolerization approaches in multiple sclerosis. Expert Opin Investig Drugs 2014; 23:9-20.
- Jelcic I, Al Nimer F, Wang J, et al: Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell 2018; 175: 85-100 e123.
- Planas R, Santos R, Tomas-Ojer P, et al: GDP-l-fucose synthase is a CD4(+) T cell-specific autoantigen in DRB3*02:02 patients with multiple sclerosis. Sci Transl Med 2018; 10.
- Sospedra M, Martin R: Immunology of multiple sclerosis. Annu Rev Immunol 2005; 23: 683-747.
- Bielekova B, Sung MH, Kadom N, et al: Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol 2004; 172: 3893-3904.
- Walczak, A, Siger M, Ciach A, et al: Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol 2013; 70: 1105-1109.
- Jurynczyk M, Walczak A, Jurewicz A, et al: Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann Neurol 2010; 68: 593-601.
- Chataway J, Martin K, Barrell K et al. Effects of ATX-MS-1467 immunotherapy over 16 weeks in relapsing multiple sclerosis. Neurology 2018; 90: e955-e962.
- Garren H, Robinson WH, Krasulova E, et al: Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann Neurol 2008; 63: 611-620.
- Zubizarreta I, Florez-Grau G, Vila G, et al: Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. Proc Natl Acad Sci U S A 2019; 116: 8463-8470.
- Lutterotti A, Yousef S, Sputtek A, et al: Antigen-specific Tolerance by Autologous Myelin Peptide-Coupled Cells – A Phase I Trial in Multiple Sclerosis. Science Translational Medicine 2013; 5: 188ra75.
- Bielekova B, Goodwin B, Richert N, et al: Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 2000; 6: 1167-1175.
InFo NEUROLOGY & PSYCHIATRY 2019; 17(6): 10-13.