Surgical therapy of renal artery stenosis (NAS) has become rare due to the advent of interventional methods, but shows very good results in terms of optimization of blood pressure control as well as stabilization of renal function. An overview of current treatment options and recommendations.
With the increasing aging of Western society, the incidence of atherosclerotic renal artery stenosis is also growing. Relevant renal artery stenosis is associated with high blood pressure, chronic renal failure, including the need for dialysis, and increased mortality [1]. Therapeutic options include drug therapy and interventional or surgical revascularization. Surgical therapy has become a rarity in recent years, but the indication for interventional therapy must also be critically examined.
In 1934, the Goldblatt experiments were the first to demonstrate the fundamental relationships between renal artery stenosis (NAS), arterial hypertension, and impaired renal function [2]. Four years later, nephrectomy successfully treated renovascular hypertension for the first time [3]. The first open-surgical kidney-preserving reconstructions were performed starting in the 1950s [4]. However, the lack of success and emerging complications dampened the initial euphoria. After improvement in diagnostic angiography, open surgical repair of a NAS has become increasingly common in hypertensive patients. However, the first published case series showed a success rate of only about 50% with respect to improvement of hypertension [5]. At that time, preoperative diagnostic resources were limited and the patient population that would benefit most from surgery was still completely unclear. In particular, research on the renin-angiotensin-aldosterone axis led to improved patient selection and, together with decades of improvements in surgical technique, to a high success rate of surgery for NAS.
The first endovascular treatment of a NAS in 1978 [6] and the first successful stent implantation of a NAS a few years later led to an exponential and euphoric increase in interventional therapy and a collapse of open surgery [7]. For example, in the United States, surgical therapy decreased from 1.3/100,000 persons in 1988 to 0.3/100,000 persons in 2009, with surgery alone for a NAS decreasing to 0.1/100,000 persons [8]. The remaining procedures (0.2/100,000) were combined renal and aortic surgeries.
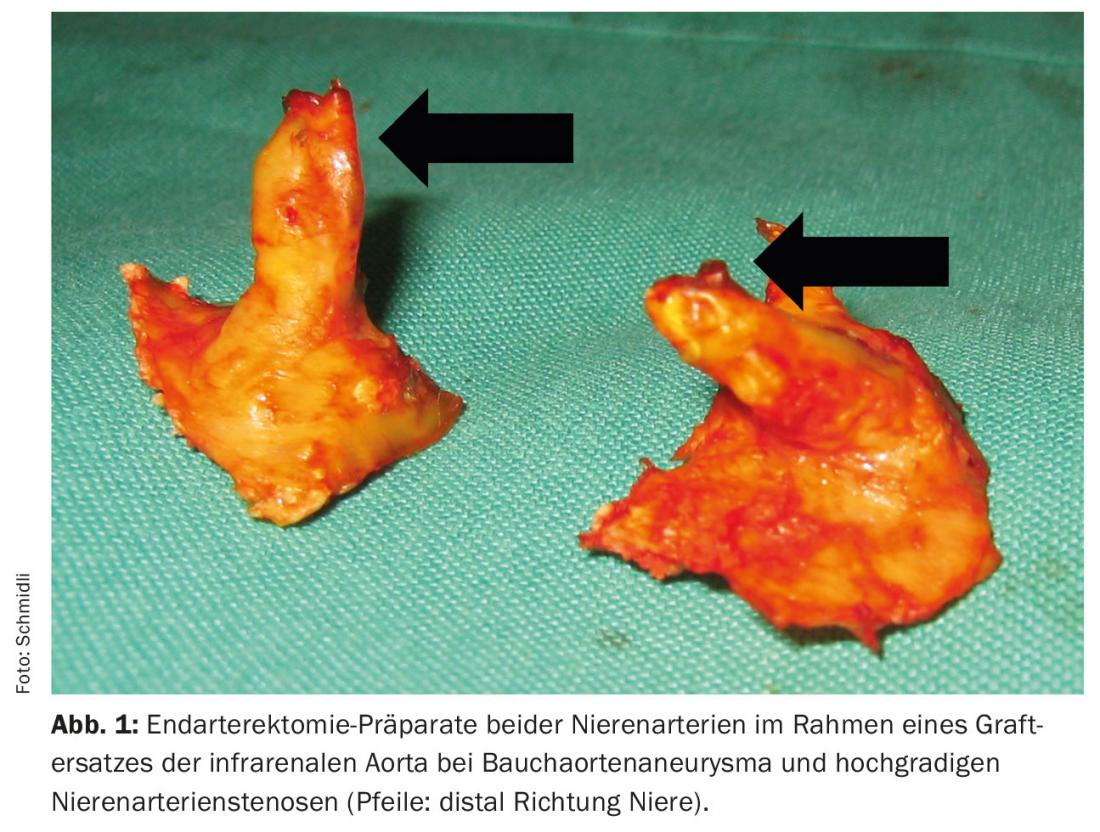
Today, surgical therapy of isolated NAS is rarely performed at our center. However, in open aortic surgery for pararenal aneurysm or occlusive disease, relevant NAS is regularly treated. Basically, there are the following three surgical treatment options: resection of the stenosis, thromboendarterectomy (TEA, Fig. 1) , and bypass grafting. In resection of the NAS, revascularization is performed either by direct reimplantation of the remaining healthy renal artery into the aorta or aortic prosthesis, respectively, or by a plastic interposition. We prefer to perform TEA of the renal artery outlets transostially (Fig. 2).
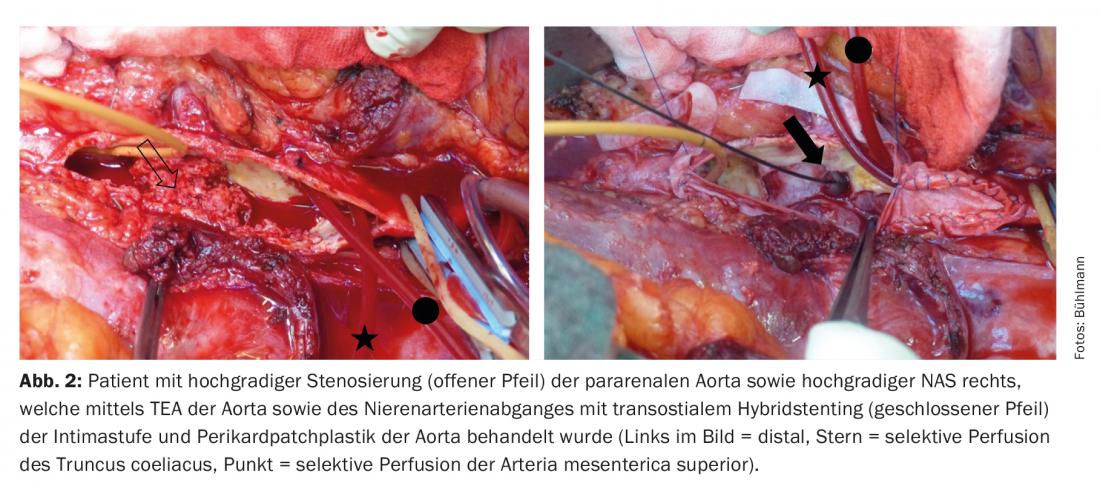
Depending on the situation, the resulting distal intimal stage can be fixed by transostial hybrid stenting under visualization (“open stenting”) (Fig. 2) . We hardly use the classic open TEA with ventral patch reconstruction at the aorta anymore because it is associated with a longer renal ischemia time. Bypass is mainly performed in isolated NAS, where we use autologous venous material especially in narrow caliber renal arteries. The renal arteries are usually extremely fragile vessels and require a fine microsurgical surgical technique with the use of magnifying glasses. All operations are performed under general anesthesia. Approaches include median or transverse laparotomy, lumbotomy, or thoracophrenolumbotomy (two-cavity procedure) for more extensive pathology, depending on the situation.
The organ ischemia resulting from the necessary vascular clamping during surgery is tolerated quite well up to 30 minutes of ischemia time. When prolonged periods of ischemia are anticipated, the use of selective crystalloid renal cold perfusion can reliably reduce the consequences of renal ischemia. The visceral ischemia time that occurs with more extensive reconstructions involving the coeliac trunk and superior mesenteric artery is reduced when a selective aortovisceral shunt or heart-lung machine is used. The hospitalization period is about one week for laparotomy/lumbotomy and about two weeks for bicavity procedures.
Renal artery surgery is a highly complex surgery and belongs in the hands of an experienced interdisciplinary treatment team, which masters the entire spectrum of open and endovascular therapy options at all times with a correspondingly high number of cases.
Open surgical treatment of renal artery stenosis has become a rarity today, and correspondingly little has been published on this topic in recent years [9]. However, what distinguishes surgery is excellent renal artery openness rates (97% at three years), whereas renal artery occlusion rates of 3% per year have been published with endovascular therapy [10]. In a large surgical series, one in nine patients no longer needed antihypertensive medication; hypertension improved significantly in 73% of patients [11]. Improvement in arterial hypertension is also significantly better when compared with endovascular therapy [10]. Renal function also improved significantly after surgical revascularization, which has not been previously shown after endovascular procedures [10]. Microemboli, which are difficult to prevent during both probing and stent-assisted balloon angioplasty, are thought to be responsible for the lack of improvement in renal function [12].
PTA of the renal artery – with or without stenting – is undoubtedly a minimally invasive procedure. The increase in endovascular therapy is often attributed to lower peri-interventional mortality (0.9% vs. 4.1%) and shorter hospital stay [8]. However, very low mortality rates of 1% for unilateral and 3% for bilateral reconstructions are reported in vascular surgery reference centers [11]. In addition, surgery no longer showed a mortality disadvantage when concurrent aortic procedures were excluded [10]. However, the great invasiveness of renal artery surgery cannot be dismissed. Perioperative morbidity is 16% [11]. The most common complications are pneumonia and cardiac complications such as arrhythmias or perioperative myocardial infarction [11].
The incidence of stent-assisted balloon angioplasty of renal artery stenosis increased from an initial 1.9/100,000 persons to 13.7/100,000 in 2006 and then decreased to 6.7/100,000 in 2009 [8].
On the one hand, the incidence of atherosclerotic NAS increases with increasing life expectancy. In patients over 66 years of age, the prevalence is 6.8%, and in patients with an elevated cardiovascular risk profile, the prevalence ranges from 10.5 to 54% [13]. On the other hand, it must be assumed that the sharp increase was mainly due to the previously generous indication. In recent years, the indication had to be questioned in many cases due to the lack of evidence in randomized trials. A recent study with systematic literature review sheds new light on the evidence of the procedures [13]. Five of seven randomized controlled trials comparing endovascular treatment of the NAS with medical therapy failed to show a significant difference in terms of improvement in arterial hypertension, mortality, renal function, renal replacement, cardiovascular events, or pulmonary edema. Quite a few study showed significant methodological flaws and are critically reviewed [9]. Some have little explanatory power due to small patient numbers. Similarly, patient selection does not appear optimal. In one study, patients with malignant hypertension were excluded, precisely the patient group that would probably have benefited most from therapy [14]. In addition, most of the included patients had on average only mild renal insufficiency and thus do not correspond to the ideal collective. In a recent retrospective analysis, patients with NAS and acute pulmonary edema and refractory hypertension combined with rapid deterioration of renal failure showed a significant reduction in mortality after interventional therapy [15]. Ultimately, this confirms our view that the patients most likely to benefit from invasive therapy must be carefully selected. It should also be remembered that progress has also been made in the primary prevention of atherosclerosis and drug therapy of arterial hypertension. Further randomized trials with a better selected cohort of patients will still have to prove the advantage over drug-only therapy.

In conclusion, the current data regarding patient selection do not support rigorous interventional or open surgical treatment of NAS, regardless of the method used. When indicated, elective surgical NAS treatment belongs in the hands of an experienced center to prevent persistent improvement of arterial hypertension and progression of renal failure. Patients with NAS should be discussed and treated in an interdisciplinary manner. Interdisciplinarity allows patient selection to be optimized, treatment quality to be assured, and any complications to be treated quickly and with the best possible outcome.
Take-Home Messages
- Surgical therapy of renal artery stenosis has become rare due to the advent of interventional methods, but shows very good results in terms of optimization of blood pressure control as well as stabilization of renal function.
- Renal artery surgery is a highly complex surgery and belongs in the hands of an experienced interdisciplinary treatment team, which masters the entire spectrum of open and endovascular therapy options at all times with a correspondingly high number of cases.
- According to the authors, the optimal patient population for surgical treatment of renal artery stenosis is younger patients with malignant or refractory arterial hypertension as well as rapid deterioration of renal function, endovascularly uncontrollable re-stenting, aortoiliac occlusive disease, or concomitant aortic aneurysms.
Literature:
- Rimmer JM, et al: Atherosclerotic renovascular disease and progressive renal failure. Ann Intern Med 1993; 118(9): 712-719.
- Goldblatt H, et al: Studies on Experimental Hypertension: I. the Production of Persistent Elevation of Systolic Blood Pressure By Means of Renal Ischemia. J Exp Med 1934; 59(3): 347-379.
- Leadbetter W, et al: Hypertension in unilateral renal disease. J Urol 1938; 39: 611-626.
- Freeman NE et al: Thromboendarterectomy for hypertension due to renal artery occlusion. J Am Med Assoc 1954; 156: 1077-1079.
- Spencer FC, et al: Diagnosis and Treatment of Hypertension Due to Occlusive Disease of the Renal Artery. Ann Surg 1961; 154: 674-697.
- Gruentzig A, et al: Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal artery stenosis. Lancet 1978; 322:801-802.
- Murphy TP, et al: Increase in Utilization of Percutaneous Renal Artery by medicare beneficiaries, 1996-2000. AJR Am J Roentgenol 2004 Sep;183(3): 561-568.
- Liang P, et al:The rise and fall of renal artery angioplasty and stenting in the United States, 1988-2009. J Vasc Surg 2013; 58(5): 1331.
- Wenk HH: Surgery of the Renal Artery: The Role of the Vascular Surgeon in Current Therapy. Zentralbl Chir 2011; 136: 422-425.
- Abela R, et al: An Analysis Comparing Open Surgical and Endovascular Treatment of Atherosclerotic Renal Artery Stenosis. Eur J Vasc Endovasc Surg 2009; 38(6): 666-675.
- Cherr GS, et al: Surgical management of atherosclerotic renovascular disease. J Vasc Surg 2002; 35(2): 236-244.
- Edwards MS et al: Atheroembolism during percutaneous renal artery revascularization. J Vasc Surg 2007; 46(1): 55-61.
- Raman G et al: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis: An Updated Systematic Review. Ann Intern Med 2016; 165(9): 635-649.
- Bax L, et al: Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: A randomized trial. Ann Intern Med 2009; 150(12): 840-848.
- Ritchie J, et al: High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 2014; 63(2): 186-197.
- Anderson JL, et al: Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 127(13): 1425-1443.
CARDIOVASC 2017; 16(3): 22-26











