The “European Society for Clinical Nutrition and Metabolism” (ESPEN) is a confederative umbrella organization of numerous national professional societies in the field of nutritional medicine. A new guideline on micronutrients in the context of clinical nutrition was published last year. Among other things, it contains numerous recommendations for clarification and supplementation of B vitamins.
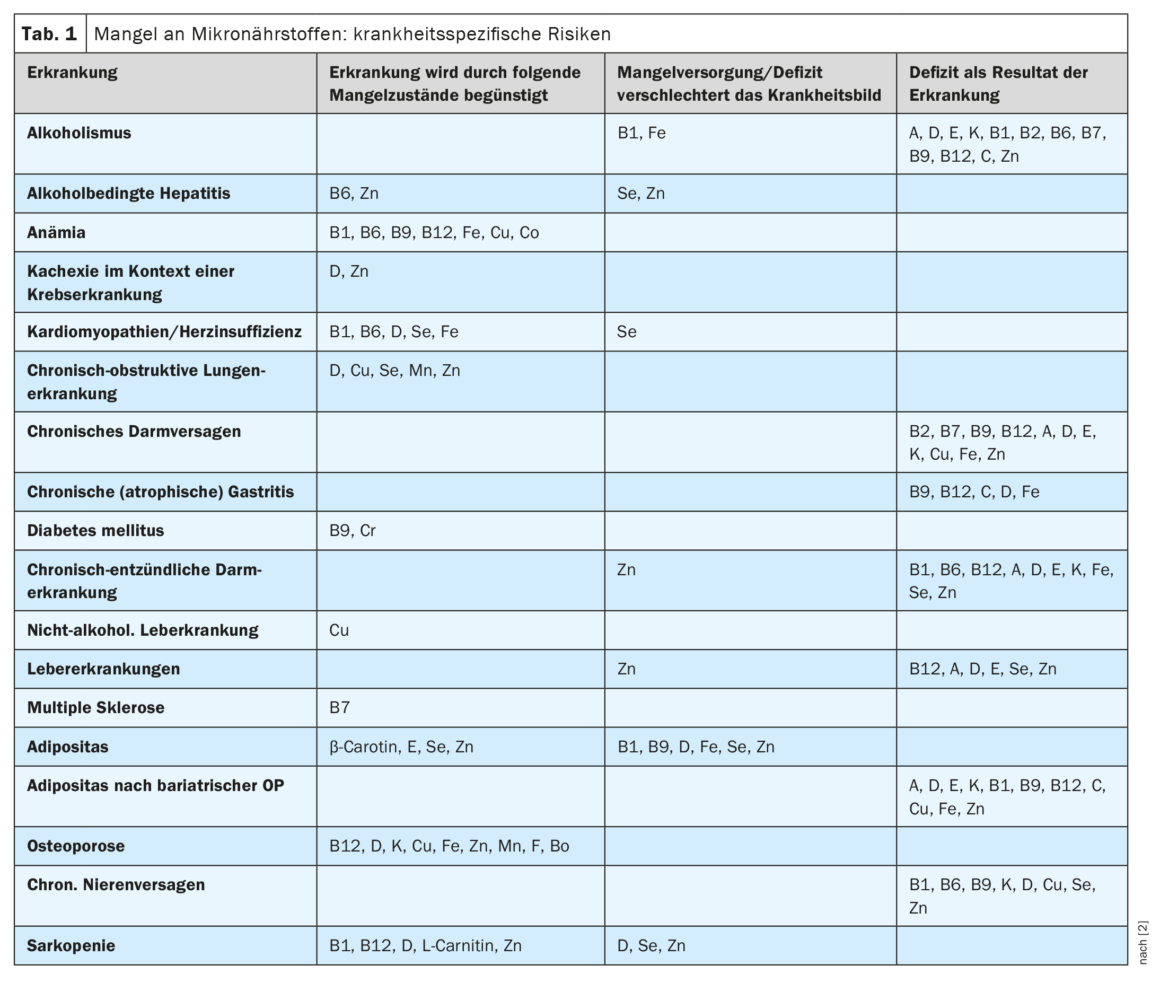
Malnutrition and undernutrition can promote the progression of diseases. In addition to the supply of macronutrients, attention must also be paid to an adequate supply of micronutrients [1]. The ESPEN guideline, published last year, provides practical recommendations to help clinicians identify patients at increased risk for deficiency symptoms (Table 1) and direct them to the appropriate supplementation regimens in the context of enteral nutrition (oral or tube feeding) or parenteral nutrition (direct infusion of small molecule nutrient solutions into the bloodstream). In addition to useful advice on risk factors for micronutrient deficiency and screening measures, specific dosing recommendations for supplementation are also provided. In the following, we will discuss some of the important points about B-complex vitamins covered in the guideline [2].
Thiamine (vitamin B1)
Thiamine is a water-soluble vitamin that is essential for carbohydrate and energy metabolism [3].
In whom is the determination of thiamine status useful?
- Thiamine can be determined in erythrocytes or whole blood in:
- Patients with suspected deficiency associated with cardiomyopathy and prolonged diuretic treatment.
- Patients undergoing nutritional evaluation as part of a prolonged medical diet and after bariatric surgery
- Refeeding Syndrome
- Encephalopathy
Thiamine status is assessed by measuring thiamine diphosphate (ThDP) in erythrocytes or whole blood. If determination of ThDP in erythrocytes or whole blood is not possible, measurement of erythrocyte transketolase and its activation by thiamine may be considered.
When should additional amounts be administered?
For patients admitted to the emergency department or intensive care unit, the guideline recommends administration of thiamine (100-300 mg/day intravenously) from admission for 3-4 days. Thiamine (100-300 mg/day) should be administered either orally or as an infusion in patients admitted to the ward with suspected decreased food intake in previous days or high alcohol consumption.
How can additional quantities be administered?
Because thiamine is well absorbed (except in alcohol-related gastritis), oral, enteral, or intravenous supplementation can be administered. However, given the severity of acute deficiency symptoms, intravenous administration at 3 x 100-300 mg per day is most efficient.
| Micronutrients subsume a pathophysiologically and functionally heterogeneous group of substances such as vitamins, minerals and trace elements. Recent research shows the importance of micronutrients in various pathologies. The vitamins of the B complex are involved in numerous metabolic reactions. B vitamins are cofactors (coenzymes) that help enzymes catalyze chemical reactions. Coenzymes are organic nonproteins that bind to their associated protein (apoenzyme) and together form a functional enzyme (holoenzyme). |
| according to [2] |
Riboflavin (vitamin B2)
Riboflavin is involved in redox reactions and antioxidant functions, in the metabolism of other B vitamins (niacin, B6, B12 and folate) and in energy production. Riboflavin is also required for normal antibody production and has several immunomodulatory effects [5]. Acute deficiency occurring during nutrition is rare unless riboflavin is not included in the micronutrient formulation or when treating high-risk patients (Table 1) . It is important to remember that riboflavin deficiency is often accompanied by deficiencies of pyridoxine, folate, and niacin.
In whom is the determination of riboflavin status useful?
Determination of riboflavin status may be required when there is a clinically reasonable suspicion of the presence of a possible deficiency. Riboflavin status can be ascertained by glutathon reductase activity in the blood count. Determination of flavin adenine dinucleotide concentration is another validated method for the determination of riboflavin, particularly in the context of inflammation. Currently, interest is focused on the role that riboflavin plays in determining circulating homocysteine concentrations, particularly in patients with polymorphisms in the MTHFR gene as a risk factor for hypertension and cardiovascular disease [6,7]. Randomized trials in hypertensive patients (with and without overt cardiovascular disease) homozygous for the MTHFR 677 TT genotype show that targeted riboflavin supplementation (1.6 mg/day) lowers systolic blood pressure independent of antihypertensive medication [8–11]. There is also evidence that high-dose riboflavin supplementation (400 mg) may be beneficial in the prophylaxis of migraine [12].
Enteral nutrition should provide at least 1.2 mg of riboflavin per day in 1500 kcal. In acute deficiency, riboflavin is administered orally at a dosage of 5-10 mg/day until recovery. Parenteral nutrition should contain 3.6-5 mg of riboflavin per day.
When should additional amounts be administered?
Additional amounts of riboflavin may be administered in the following cases:
- Suspected or proven clinical deficiency
- Patients at risk of deficiency
- In patients with a deficiency of other B-complex vitamins.
- in patients with myoadenylate deaminase deficiency, as some of them are sensitive to this cofactor
How can additional quantities be administered?
In case of deficiency, 5-10 mg/day of riboflavin can be taken orally. Clinical riboflavin deficiency may require intravenous administration of 160 mg riboflavin for four days. In patients with multiple acyl-CoA dehydrogenase deficiency (MADD), riboflavin can be administered in doses of 50-200 mg/day.
Niacin (vitamin B3)
Niacin is a collective term for nicotinic acid and nicotinamide. All tissues in the body convert ingested niacin into its major metabolically active form, the coenzyme nicotinamide adenine dinucleotide (NAD). More than 400 enzymes require NAD to catalyze reactions in the body.
In whom is the determination of niacin status useful?
In the presence of clinical symptoms such as diarrhea, dermatitis, and dementia (pellagra disease), NAD levels can be measured in blood or tissue. Alternatively, with a stored blood sample, one can wait to see the effects of niacin supplements on symptoms. DNAD in blood or tissue can be used as a measure of niacin status.
When and how should additional amounts be administered?
Higher doses may be required if niacin deficiency is suspected due to a high-risk clinical history and/or the presence of signs or symptoms. Oral/enteric administration should always be done when the gastrointestinal tract is functional. In cases of malabsorption and short bowel, the parenteral route can be chosen.
Pantothenic acid (vitamin B5)
Pantothenic acid is a component of coenzyme A (CoA) and acyl carrier protein (ACP) and therefore involved in numerous biochemical processes of oxidative respiration, lipid metabolism, synthesis of steroids, acetylated molecules (amino acids, carbohydrates), and prostaglandins [14].
In whom is the determination of pantothenic acid status useful?
The guideline recommends determining pantothenic acid in the blood. Possible pantothenic acid deficiency should be thought of in patients with neurological symptoms. There is evidence of associations with Huntington’s disease and Alzheimer’s disease.
Enteral nutrition should provide at least 5 mg of pantothenic acid per day at an intake of 1500 kcal. Parenteral nutrition should provide at least 15 mg of pantothenic acid per day.
When and how should additional amounts be administered?
In the context of atypical neurological symptoms, additional pantothenic acid may be administered along with other B vitamins.
Pyridoxine (vitamin B6)
The name vitamin B6 refers to a group of six water-soluble pyridine compounds (B6 vitamers) that include pyridoxine, pyridoxamine, pyridoxal, and their respective phosphorylated forms [15]. The biologically active form of vitamin B6 is pyridoxal phosphate, which serves as a coenzyme for more than 160 enzymatic reactions. These reactions include transamination, racemization, decarboxylation, and aldol cleavage, which affect carbohydrate, protein, and lipid metabolism [15]. The major function of active phosphorylated vitamin B6 in the cell is related to both biosynthesis and degradation of amino acids and is central to transamination reactions [16]. Other functions include gluconeogenesis (via glycogen phosphorylase), binding to steroid receptors, neurotransmitter synthesis, and heme biosynthesis.
In whom is the determination of pyrodixin status useful?
If signs of pyridoxine deficiency are present, vitamin B6 status can be determined by measuring plasma pyridoxal phosphate (PLP) levels. In critically ill patients or in cases of inflammation, PLP should be measured in erythrocytes.
Enteral nutrition should provide at least 1.5 mg of pyridoxine per day in 1500 kcal. Parenteral nutrition should provide 4-6 mg of pyridoxine per day.
When and how should additional amounts be administered?
In the context of isoniazid overdose or glycol poisoning, a high dose of pyridoxine should be part of the therapy.
Biotin (vitamin B7)
Biotin is found in all cells of the human body. It plays an important role in the metabolism of fatty acids, glucose and amino acids, being a cofactor for five carboxylases that are crucial for their metabolism [17,18]. Biotin is also a regulator of gene expression and influences the functions of T cells and natural killer cells of the adaptive immune system [16]. Adequate biotin levels are also essential for normal fetal development.
In whom is the determination of biotin status useful?
Conditions with an increased risk of developing biotin deficiency include alcohol consumption, malabsorption in the context of inflammatory bowel disease, irritable bowel syndrome, celiac disease, severe malnutrition, smoking, and pregnancy. Long-term use of antibiotics can destroy microorganisms that produce biotin. In addition, there are study findings suggesting biotin deficiency associated with lack of supplementation of parenterally fed patients, and there is evidence that long-term use of anticonvulsants is associated with poorer absorption and increased requirement for biotin [2].
Biotin status can be obtained in the presence of clinical symptoms suggestive of biotin deficiency (e.g., dermatitis, hair loss, or neurologic symptoms) and a history suggestive of inadequate intake. Biotin status is determined by direct measurement of biotin in blood and urine and should be supplemented by determination of biotinidinase activity.
In enteral nutrition, at least 30 μg of biotin per day should be supplied in 1500 kcal. For parenteral nutrition, vitamin supplements should provide 60 μg of biotin per day.
When and how should additional amounts be administered?
Nursing mothers should receive an oral intake of at least 35 μg biotin per day. Supplementation may also be required in patients on renal replacement therapy. Additional amounts of biotin can be administered either orally, enterally, or intravenously, depending on intestinal function.
Folate and folic acid (vitamin B9)
Folate is a generic term that refers to a family of molecules that vary depending on their oxidation state, the chemical nature of the C1 units, and the length of the glutamate side chain [20].
In whom is the determination of vitamin B9 status useful?
In patients with macrocytic anemia or at risk of malnutrition, folic acid status should be measured at least once at initial screening and repeated within three months of supplementation to verify normalization. Folic acid and B12 are usually both measured when testing for anemia. For diseases known to increase the need for folic acid, folic acid status can be measured every 3 months until stabilization and then once a year.
Folic acid status should be determined in plasma or serum (short-term status) or in erythrocytes (long-term status) using a method validated against the microbiological assay. The gold standard method for measuring folate is the microbiological test using L. rhamnosus. Simultaneous analysis of homocysteine improves interpretation of laboratory measurements. Step A is supported by biochemical evidence.
Enteral nutrition should provide 330-400 μg dietary folate equivalent (DFE) per day in 1500 kcal. Parenteral nutrition should provide 400-600 μg of folic acid per day.
When and how should additional amounts be administered?
In cases of dietary deficiency or chronic hemodialysis, 1-5 mg of folic acid per day can be administered orally. In case of deficiency, oral administration should be given for four months, or until the cause of the deficiency is corrected. When clinical symptoms have resolved and blood counts have normalized, a maintenance dose should be administered, i.e., approximately 330 μg DFE (dietary folate equivalent) for adults and 600 μg DFE for pregnant and lactating women, per day.
In chronic hemodialysis patients with hyperhomocysteinemia, higher amounts may be required over longer periods: 5 mg or more of folic acid per day in nondiabetic patients and 15 mg per day in diabetic patients [21,22].
To prevent neural tube defects, women of childbearing potential or those not taking oral contraceptives and living in countries where staple foods are not fortified with folic acid should take folic acid supplements (400 μg/day) periconceptually/at childbearing age. It is recommended to take additional amounts of folic acid orally. In case of ineffective oral treatment or intolerance, folic acid (0.1 mg/day) may alternatively be administered subcutaneously, intravenously, or as an infusion
Cobalamin (vitamin B12)
Vitamin B12 (cobalamin) is an essential water-soluble micronutrient synthesized by fungi and microorganisms and in the stomach of ruminants depending on the cobalt content of the soil [23].
In whom is the determination of cobalamin status useful?
Cobalamin deficiency should be excluded in all patients who have anemia or isolated macrocytosis and have been diagnosed with polyneuropathies, neurodegenerative diseases, or psychosis. In all high-risk patients or patients being treated with cobalamin, the adequacy of intake should be reviewed at least annually based on resolution of clinical symptoms and available laboratory markers.
Adult patients at risk or suspected of cobalamin deficiency should be evaluated with the combination of at least two biomarkers (holotranscobalamin, methylmalonic acid), with serum cobalamin as a surrogate. Patients with autoimmune diseases or with glossitis, anemia, and neuropathy should be evaluated for pernicious anemia in the presence of anti-intrinsic factor antibodies regardless of cobalamin levels.
Enteral nutrition should provide at least 2.5 μg of cyanocobalamin per day in 1500 kcal. Parenteral nutrition should provide at least 5 μg of cyanocobalamin per day.
When and how should additional amounts be administered?
For nursing mothers, an oral intake of at least 2.8 μg cyanocobalamin per day is recommended. Patients with impaired cobalamin absorption should receive lifelong supplements, either in the form of a daily dose of 350 μg cobalamin or as an IM injection of 1000-2000 μg cobalamin every 1-3 months. Intranasal and sublingual administration are alternative routes [19].
In cases of acute clinical symptoms of deficiency, antibodies to intrinsic factor, a history of total gastrectomy, or continuous malabsorptive disease, administration should be by infusion, starting with high doses of 1000 μg cobalamin every other day for two weeks (or daily for five days).
Treatment should be continued at least twice monthly until all clinical symptoms and/or etiopathogenetic factors (including macrocytosis) have resolved. Blood potassium monitoring should be performed as part of saturation therapy.
Literature:
- Hauner, H et al: Leitfaden Ernährungstherapie in Klinik und Praxis (LeKuP). In: Current Nutritional Medicine 2019; 44: 384-419.
- Berger MM, et al: ESPEN micronutrient guideline. Clin Nutr 2022; 41(6): 1357-1424.
- Johnson CR, et al: Thiamine deficiency in low- and middle-income countries:disorders, prevalences, previous interventions and current recommendations. Nutr Health 2019; 25: 127e51.
- “Thiamine deficiency and its prevention and control in major emergencies”. Geneva: WHO; 1999. www. who.int/publications/i/item/WHO-NHD-99.13,(last accessed 20.03.2023).
- Packer M: Cobalt cardiomyopathy: a critical reappraisal in light of a recent resurgence. Circ Heart Fail 2016: 9.
- Powers HJ: Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77: 1352e60.
- McNulty H, et al: Riboflavin, MTHFR genotype and blood pressure: a personalized approach to prevention and treatment of hypertension. Mol Aspect Med 2017; 53: 2e9.
- Horigan G, et al: Riboflavin lowers blood pressure in cardiovascular disease patients homozygous for the 677C->T polymorphism in MTHFR. J Hypertens 2010; 28: 478e86.
- Wilson CP, et al: Riboflavin offers a targeted strategy for managing hypertension in patients with the MTHFR 677TT genotype: a 4-y follow-up. Am J Clin Nutr 2012; 95: 766e72.
- Wison CP, et al: Blood pressure in treated hypertensive individuals with the MTHFR 677TT genotype is responsive to intervention with riboflavin: findings of a targeted randomized trial. Hypertension 2013; 61: 1302e8.
- Psara E, Pentieva K, Ward M, McNulty H: Critical review of nutrition, blood pressure and risk of hypertension through the lifecycle: do B vitamins play a role? Biochimistry 2020; 173: 76e90.
- Schoenen J, Jacquy J, Lenaerts M: Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology 1998; 50: 466e70
- Institute of Medicine (IOM) Standing Committee. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. In: (US) NAP, editor. Dietary reference intakes for thiamine, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC); 1998.
- Trumbo P: Pantothenic acid. In: AC R, B C, Cousins RJ, Tucker KL, Ziegler TR (Eds). Modern nutrition in health and disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014. P. 351e7
- Oppici E, et al: Pyridoxamine and pyridoxal are more effective than pyridoxine in rescuing folding-defective variants of human alanine:glyoxylate aminotransferase causing primary hyperoxaluria type I. Hum Mol Genet 2015; 24: 5500e11.
- Parra M, Stahl S, Hellmann H: Vitamin B(6) and its role in cell metabolism and physiology. Cells 2018: 7.
- Agrawal S, Agrawal A, Said HM: Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol 2016; 311: C386e91.
- Gifford JL, de Koning L, Sadrzadeh SMH: Strategies for mitigating risk posed by biotin interference on clinical immunoassays. Clin Biochem 2019; 65: 61e3.
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010;26:1031e7
- Scaglione F, Panzavolta G: Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014; 44: 480.
- Capelli I, et al: Folic acid and vitamin B12 administration in CKD, Why Not? Nutrients 2019: 11.
- Angelini A, et al: The link between homocysteine, folic acid and vitamin B12 in chronic kidney diseases. G Ital Nefrol 2021; 38: 2021.
- Jarquin Campos A, et al: Diagnostic accuracy of holotranscobalamin, vitamin B12, methylmalonic acid, and homocysteine in detecting B12 deficiency in a large, mixed patient population. Dis Markers 2020; 2020: 7468506.
HAUSARZT PRAXIS 2023; 18(4): 15-19