At the DGHO Congress in Vienna, various aspects in clinical practice in non-small cell lung cancer (NSCLC), renal cell cancer (RCC) and chronic myeloid leukemia were addressed. In particular, the current research situation on the new tyrosine kinase inhibitors crizotinib, sunitinib and bosutinib was discussed.
Non-small cell lung cancer (NSCLC) is usually asymptomatic for a long period of time. More than half of the patients are already at an advanced stage when they are first diagnosed.
“Drug therapy for metastatic NSCLC is currently not very effective, and the classic cytostatic drugs have been exhausted. The survival time in current studies without biologics is 10-12 months,” said Prof. Norbert Frickhofen, MD, Wiesbaden, Germany. “However, the genetics are well understood. Translocated ALK is a potent oncogene and a key factor in carzigonesis in a proportion of NSCLC patients. Mostly it occurs with the fusion partner EML4, besides there are rarer partners such as KIF5B and TFG. Therefore, in NSCLC with ALK fusion gene, targeted inhibition is a good therapeutic option because there is constitutive activation of ALK kinase by the described fusion.”

Crizotinib causes such an inhibition of the tyrosine kinase ALK. It is an oral aminopyridine that also blocks MET and ROS1. It is approved in Switzerland as Xalkori®. The FDA cleared it in 2011 for locally advanced or metastatic, ALK-positive (evaluated by an FDA-approved test) NSCLC. The EMA approved it in 2012 as a therapy for pretreated locally advanced or metastatic ALK-positive NSCLC.
A phase III trial with a primary endpoint of progression-free survival (RECIST 1.1, independent radiologic review) evaluated efficacy after prior therapy (Fig. 1) [1]. It concludes that crizotinib is superior to standard chemotherapy in patients with previously treated ALK-positive NSCLC. Median progression-free survival was 7.7 months in the crizotinib (n=173) and three months in the chemotherapy group (n=174), and the results were statistically significant (p<0.001). Response rates were also significantly higher with crizotinib (65 vs. 20%, p<0.001) and median time to response was faster (6.3 vs. 12.6 weeks). However, an interim analysis showed no significant improvement in overall survival. The most common side effects were visual disturbances, gastrointestinal side effects, and elevated liver aminotransferase levels. Overall, patients taking crizotinib vs. chemotherapy noticed a greater reduction in lung cancer symptoms and a greater improvement in overall quality of life.
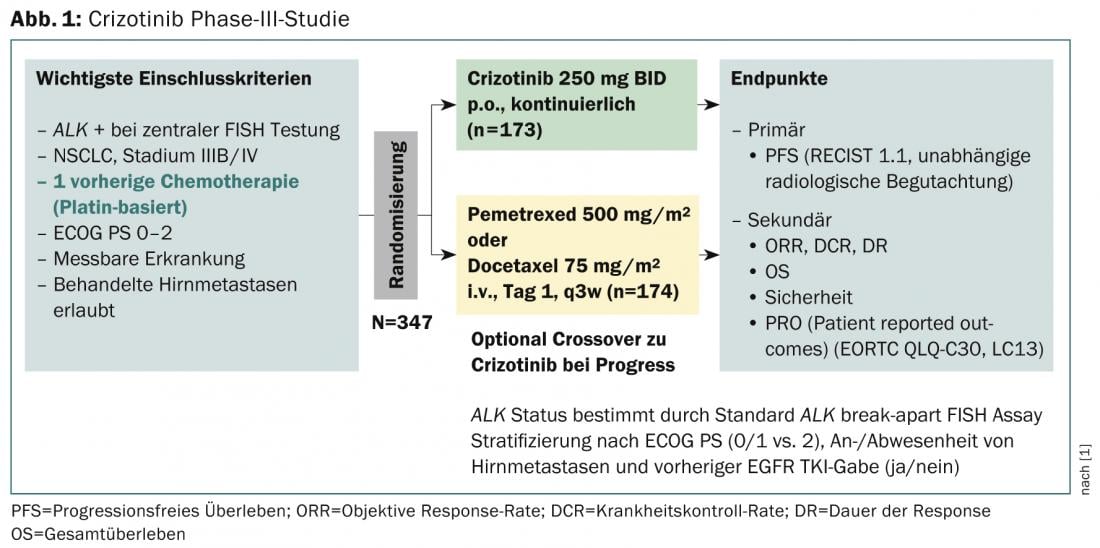
Crizotinib in practice
In practice, the question is: Who should be tested for ALK? “The answer is any patient for whom ALK inhibition is a therapeutic option. ALK diagnosis proceeds via IHC screening and FISH screening. Regarding side effects, visual disturbances such as tracers in the peripheral visual field in borderline light, drooping of objects, flashes of light without reference, or contrast reversals are functional and not critical. The most critical side effect is hepatotoxicity, reaching grade 3/4 in 16%, which can result in death and therefore must be carefully controlled and rapidly addressed. If there is an increase in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) grade 3 or 4 and total bilirubin ≤grade 1, discontinuation is recommended until recovery to ≤grade 1 or baseline, then restart with 2×200 mg/d. If total bilirubin also increases to grade 2, 3, or 4 (in the absence of cholestasis or hemolysis), discontinuation of therapy is necessary,” said Prof. Frickhofen. “Overall, specific therapy with crizotinib is effective and tolerable in ALK-positive NSCLC.”
Options in mRCC
“The treatment options for metastatic renal cell carcinoma must be optimally exploited. Specifically, this means consistent treatment with one substance for as long as possible and achieving the best possible remission. In addition, the second line should be considered already in the first line therapy. The management of side effects must be optimized and the education and involvement of the patient and the co-treating physicians (general practitioners) must be promoted,” says PD Dr. med. Jochen Casper, Oldenburg.
For first-line, sunitinib, bevacizumab plus IFN, and pazopanib may be considered if there is clear cell histology and a good/intermediate prognosis. Although not in a head-to-head comparative study, Dr. Casper showed that the three factors of progression-free and overall survival, as well as response, tended to be better with sunitinib (Sutent®) than with the other treatment options, based on several randomized phase III trials in low- and intermediate-risk settings. A retrospective analysis [2] on sunitinib also concludes that response correlates with overall survival. It was significantly higher in responders, and long overall survival was also seen in late responders.
“Not only is tumor shrinkage related to overall survival in both first- and second-line settings, but response and the extent of response are also relevant to this. This effect, as far as studied, is independent of the therapy used (tyrosine kinase inhibitor [TKI], mTOR, cytokine). The inclusion of first-line therapy or the correct sequence in the choice of second-line can have a significant impact on overall survival. Further studies in this area are needed. However, the goal must always be overall survival, and this has improved significantly since the introduction of TKIs,” Dr. Casper concluded.
Optimize TKI therapy also in CML
PD Philipp le Coutre, MD, Berlin, spoke about chronic myeloid leukemia (CML). A new tyrosine kinase inhibitor approved by the FDA in 2012 for the treatment of CML is called bosutinib. It is used in patients in whom other TKIs do not work or are poorly tolerated. In Europe, the active ingredient was conditionally approved by the European Commission in 2013 as a drug for the treatment of an orphan disease (not yet approved in Switzerland).
“This new therapeutic option could offer a potential alternative in cases of inadequate response and resistance, specific patient comorbidities or risk factors, and intolerance due to prior therapies,” Dr. le Coutre concluded the presentation.
Source: “Evidence and clinical practice in NSCLC, RCC and CML”, Pfizer Pharma GmbH satellite symposium at the DGHO Congress, October 18-22, 2013, Vienna.
Literature:
- Shaw AT, et al: Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385-2394.
- Molina AM, et al: Sunitinib objective response in metastatic renal cell carcinoma: analysis of 1059 patients treated on clinical trials. Eur J Cancer 2013 Sep 16. pii: S0959-8049(13)00789-2. doi: 10.1016/j.ejca.2013.08.021. [Epub ahead of print].
InFo Oncology & Hematology 2014; 2(2): 36-38.











