At the end of 2013, the 55th annual congress of the “American Society of Hematology” took place. Over 22,000 participants came to New Orleans, or “The Big Easy” as the city is known. Interested parties can find the complete program with excerpts of the presentations at www.hematology.org.
Haploidentical stem cell transplantation
(mb) HLA haploidentical hematopoietic stem cell transplantation is an effective option for patients who need a transplant but cannot find a donor with completely matching HLA types. This is the summary of Alice Bertaina, MD, Bambino Gesu Children’s Hospital, Rome. Such treatment, compared with stem cell transplantation from a completely matched donor, is usually associated with a higher risk of infection and relapse. In the method demonstrated by Bertaina, the Alfa/Beta+ T cells as well as the CD19+ B cells are selectively removed from the donor graft. At the same time, one retains immune active cells as natural killer cells and gamma/delta+ T cells. A total of 45 children with acute leukemia – aged between 0.9 to 17.9 years during transplantation – were treated in this way with stem cells from a parent (35 patients with ALL and 10 with AML). The median time to achieve an absolute neutrophil count of >0.5 × 109/l and a platelet count of >50 × 109/l was 13 (9-18) and 11 days (8-20), respectively. No child developed acute graft-host reaction (GVHD) in the intestine or liver. Mild GVHD occurred in the skin of 29%. After follow-up of an average of eleven months (between 2 and 30), the two-year Kaplan-Meier estimate was a leukemia-free survival rate of 75% (95% CI 57-86). This value was 73% (95% CI 52-85) for ALL patients. “Our results with haploidentical donor stem cells herewith showed percent success rates that were consistent with those of a full-match transplant,” Dr. Bertaina explained. “Thus, this life-saving treatment becomes available to a many larger number of patients without a perfect donor match.”
Chimeric antigen receptor (CAR)
Several researchers presented another technical tour de force: a therapy using CAR-modified T lymphocytes for oncohematological diseases. The chimeric antigen receptor (CAR) is an artificially constructed DNA chain that is incorporated into a patient’s T cells using vectors (known from molecular genetics). These “new” cells combine the specificity of antibodies with the cytotoxic effect of T cells.
Michael Kalos of the University of Pennsylvania Perelman School of Medicine in Philadelphia presented results of trials in advanced relapsed or refractory CLL and ALL disease (Porter et al NEJM 2011; Kalos et al Sci Trans Med 2011, Grupp et al NEJM 2013, abstract 67). A total of 32 heavily pretreated CLL patients were treated, with eight patients having partial remission and seven having complete remission. The results can be found in Table 1.
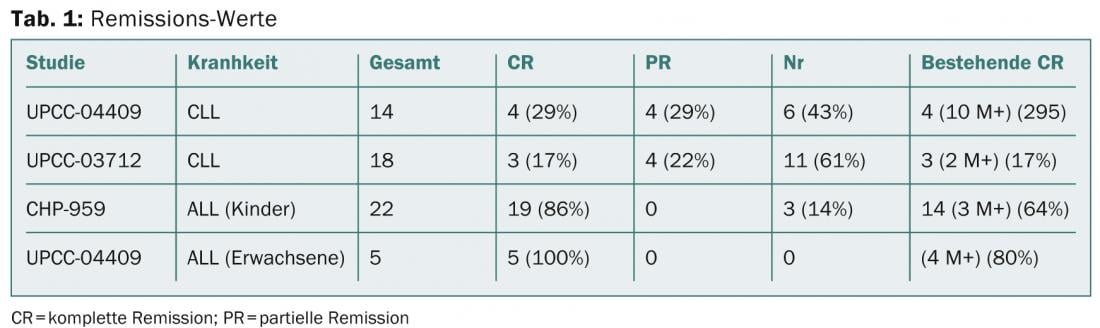
CAR in B-cell lymphoma
That patients with chemotherapy-refractory primary mediastinal B-cell lymphoma (PMBCL) and diffuse large B-cell lymphoma (DLBCL) can also benefit from anti-CD19 CAR therapy is demonstrated by the report of James Kochenderfer, MD, of the National Cancer Institute of the National Institute of Health in Bethesda. The researchers treated 20 patients with a total of 23 T-cell infusions. The first nine CAR-T cell treatments have been reported previously (Kochenderfer et al. in Blood 2010 and Blood 2012). At ASH, Kochenderfer now presented the unreported results of 14 patients. Because prior chemotherapy made clear that adoptively transferred T cell activity improves, patients receive cyclophosphamide plus fludarabine for five days for an infusion of anti-CD19 CAR T cells. Five patients experienced complete remission (CR) and six experienced partial remission (PR).
“This is the first report of successful treatment of chemotherapy-refractory PMBCL and DLBCL with anti-CD19 CAR T cells,” Kochendorfer said. “Our data are the first to suggest the potential of this approach for patients with aggressive lymphomas that have been nearly untreatable.”
Zevalin and rituximab in comparison
Consolidation therapies with Zevalin (90Y ibritumomab-tiuxetan) and rituximab both demonstrated substantial progression-free survival (PFS) benefit. A Spanish phase II study clarified that consolidation therapy with rituximab is superior to consolidation with Zevalin in patients with follicular lymphoma who respond to R-CHOP. From June 2008 to July 2010, 146 patients (66 men and 80 women with an average age of 55 years) from 25 Spanish institutions were included. Armando Lopez-Guillermo, MD, of Hospital Clinic de Barcelona, presented the preliminary results. After follow-up of an average of 37 months from randomization (range 26 to 56), 31 patients showed advanced progression or recurrence. The 36-month PFS was 64% (95% CI 52-76) for patients in the Zevalin group and 86% (95% CI 77-95) for patients in the rituximab group (p=0.01; HR 0.38, 95% CI 0.170.83). During the maintenance period, neutropenia (grade 3-4) occurred in six of the 63 patients in the Zevalin group and thrombocytopenia (3-4) in five of them. For patients in the rituximab group, it was one or none of the 61 patients. Five patients died during follow-up due to progressive lymphoma. No difference was found between the groups.
Bortezomib in multiple myeloma.
In multiple myeloma (MM), bortezomib induction plus maintenance treatment continues to improve survival of newly diagnosed symptomatic patients. This is the result of the hOVON65 study, the long-term results of which were presented by Pieter Sonneveld, MD, of the Erasmus Medisch Centrum, Rotterdam. In this study, 827 MM patients were randomized to VAD (vincristine, doxorubicin, dexamethasone; n=414) or PAD (bortezomib, doxorubicin, dexamethasone; n=413) after induction therapy, followed by high-dose melphalan and autologous stem cell transplantation. Maintenance therapy consisted of 50 mg thalidomide daily (for the VAD group) or 1.3 mg bortezomib/m2 iv biweekly (for the PAD group) for two years. Response during protocol treatment appeared slightly improved now that all patients have completed treatment: Complete remission (CR) plus nodular complete remission (nCR) occurred in 49% with PAD and 35% with VAD. Very good partial remission (VGPR) was seen in 26% and 21%, respectively, and ≥ partial remission (PR) was seen in 91% and 83%, respectively. After follow-up of an average of 67 months, 111 of the patients treated with VAD and 131 of those treated with PAD were progression-free and alive. Progression-free survival (the time from randomization to progression, recurrence, or death) was better with PAD (stage-adjusted) (ISS) (HR 0.78; 95% CI 0.66-0.91; p=0.002). PAD was also superior for the secondary outcome of overall survival after ISS adjustment (HR 0.80; 95% CI 0.65-1.00; p=0.047).
Novel mutation in JAK-2-negative ET and PMF.
Austrian and Italian researchers have discovered a new mutation specific to patients with JAK-2-negative essential thrombocythemia (ET) and primary myelofibrosis (PMF). The most common genetic alteration in myeloproliferative syndrome (MPN) is the JAK2-V617F mutation. This occurs in 95% of patients with polycythaemia vera (PV) and in 50-60% of patients with ET and PMF. Mutations in exon 12 of JAK2 and in the thrombopoietin receptor gene MPL are present in an additional 5-10% of cases. In recent years, it has become apparent that several other genes are affected in MPN. However, these mutations are also found in other myeloid diseases. A specific molecular marker for the remaining 40% of ET and PMF patients with wild-type JAK2 and MPL is therefore very welcome.
Thorsten Klampfl of the Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, and his colleagues identified novel mutations in PMF patients with wild-type JAK2 and MPL using whole exome sequencing. Analysis revealed recurrent somatic insertions and deletions in CALR coding for calreticulin. All mutations detected resulted from a reading frame shift and were clustered in the last exon (exon 9) of the gene. Following this discovery, the researchers developed a PCR-based assay that they used to screen 1,107 MPN patients for insertion/deletion mutations in exon 9 of CALR. No mutations were found in PV patients, but mutations were found in ET and PMF patients: the CALR mutations were mutually exclusive with mutated JAK2 and mutated MPL. Among patients with wild-type JAK2 and MPL, 67% of ET and 88% of PMF patients had mutant CALR. No CALR exon 9 mutations were detected in 254 patients with de novo AML, in 45 with chronic myeloid leukemia, in 73 with myelodysplastic syndrome, or in 64 with chronic myeloid leukemia.
The authors anticipate that the marker will soon be available to clinicians to improve diagnostic and therapeutic decisions in MPN.
Source: 55th ASH Annual Meeting, December 7-10, 2013, New Orleans.
InFo Oncology & Hematology 2014; 2(3): 30-32.
Congress Special 2014; 5(2): 6-7











