Oral Janus kinase inhibitors (JAKi) are gaining ground in the treatment of inflammatory rheumatoid diseases and were therefore a hotly debated topic at this year’s annual congress of the Swiss Society of Rheumatology (SGR). Prof. Dr. med. Andrea Rubbert-Roth and Prof. Dr. med. Gerd Burmester provided information on current developments.
“What you always wanted to know about JAKi”. This was the title of the AbbVie Satellite Symposium on September 8 in Interlaken, moderated by Prof. Andrea Rubbert-Roth, MD, St. Gallen, and Prof. Gerd Burmester, MD, Berlin. The participants did not expect a classic frontal lecture, but an interactive “dice game”. An oversized fabric cube decided which main topic around JAKi should be discussed; the participants then voted on the specific question to the expert. A particular focus was on the selective JAK inhibitor upadacitinib (UPA; RINVOQ®), which is the only JAKi in Switzerland that can currently be used in the three indications ankylosing spondyloarthritis (AS), and psoriatic arthritis (PsA) and rheumatoid arthritis (RA) [1-3].

Prof. Andrea Rubbert-Roth, MD, and Prof. Gerd Burmester, MD, at the SGR Congress 2022.
Are cardiovascular events a class effect of JAKi?
The first roll: a 6 – Joker question! The caster from the audience wants to know, “Are cardiovascular events a general problem of JAKi?” Prof. In this regard, Burmester first discusses the prospective, randomized, open-label, post-marketing ORAL Surveillance Study, which evaluated the non-inferiority of the JAKi tofacitinib versus the tumor necrosis factor inhibitors (TNFi) etanercept or adalimumab (ADA) with respect to the occurrence of major adverse cardiovascular events (MACE) and malignancies in 4,362 patients over 50 years of age with rheumatoid arthritis (RA) and at least one other cardiovascular risk factor. As a result, tofacitinib showed a higher risk of MACE and malignancies than comparator TNFi [4]. Is this now a class effect of JAKi? “We know that JAKi have different properties, including their half-life, metabolization and selectivity,” Prof. Burmester said. In addition, he said, there are several important points to consider when interpreting ORAL surveillance data, such as the very high disease activity, the often long disease duration, and the mostly suboptimal pretreatment of the largely U.S. patient population. Whether these results are transferable to European populations needs to be investigated in further analyses, e.g., with registries.
Discuss therapy goals and align expectations at the start of treatment
Next, a practical question comes into focus: “What important points do you discuss with patients when starting therapy?” Prof. Rubbert-Roth points out possible discrepancies between patient and physician with regard to the therapy goal. “Important parameters for patients are mainly pain, functional capacity, fatigue and sleep. Physicians, on the other hand, tend to focus more on objective measures, such as inflammatory parameters and imaging [5-10].” Prof. Burmester adds, “Over the years, patient-reported outcomes (PROs) have become increasingly important for me as well. With the fitness watches available today, we can objectively measure simple parameters such as sleep quality or daily number of steps.” In addition, patients’ expectations of therapy success have increased significantly, according to Prof. Burmester: “In the past, it was important not to end up in a wheelchair. Today, the main goal is to be able to play a third set of tennis. We have to respect these high expectations and try to meet them.”
Safety profile of UPA in relation to serious infections and malignancy.
“What about severe infections under UPA?” participants now want to know. Prof. Rubbert-Roth shows the results of an integrated safety analysis of UPA in RA, AS and PsA (Figure 1). Their conclusion was that rates of serious infections and opportunistic infections were generally similar across treatment groups within each indication. Prof. Burmester highlights the good risk profile especially in AS patients [11]. With regard to increased rates of herpes zoster among UPA, he also makes a case for the Shingrix® vaccine, which he says can largely minimize the risk of herpes zoster. Also, in terms of malignancy, the integrated safety analysis showed no significant differences between UPA and ADA or methotrexate (MTX) in RA and PsA, respectively [11]. “We are quite comfortable with the assumption that there is no signal here. In AS, which often affects younger patients, malignancy rates are even lower than in RA and PsA,” comments Prof. Rubbert-Roth.
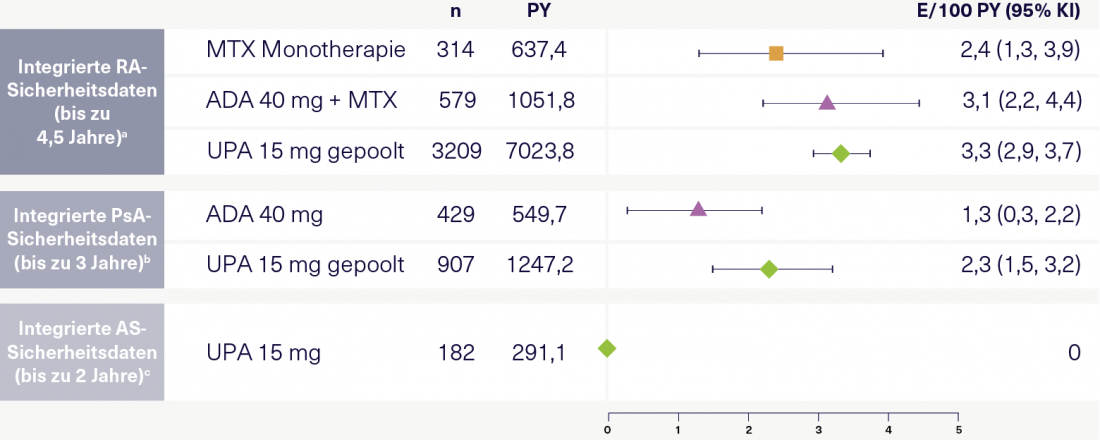
Figure 1: Serious infections per 100 patient-years in RA, PsA, and AS according to the integrated UPA safety analysis. Adapted according to [11].
a Includes 6 randomized UPA-RA trials from the SELECT trial program. Data were pooled and grouped according to treatment and/or dosing regimen. b Patients with inadequate response to or intolerance of ≥ 1 nonbiologic DMARD (SELECT-PsA 1) or ≥ 1 biologic DMARD (SELECT-PsA-2). c patients from SELECT-AXIS 1. ADA, adalimumab; AS, ankylosing spondylitis; bDMARD, biologic disease-modifying antirheumatic drug; CI, confidence interval; E, event; MTX, methotrexate; PsA, psoriatic arthritis; PY, patient-years; RA, rheumatoid arthritis; UPA, upadacitinib.
When can UPA be used as monotherapy?
“Quite a number of patients are increasingly complaining of intolerance to MTX and no longer want to take it,” Prof. Burmester reports. Effective and tolerable monotherapy is therefore becoming increasingly attractive. In the randomized, double-blind, phase III SELECT-MONOTHERAPY trial, RA patients with inadequate response to MTX on UPA monotherapy (15 mg, 1x daily) achieved a DAS28-CRP remission rate (<2.6) of 28% at 14 weeks, compared with 8% on MTX monotherapy (p ≤ 0.0001) [12]. In the randomized, double-blind, phase III SELECT-COMPARE trial, remission rates at 12 weeks were 29% with UPA (15 mg, 1x daily) + MTX, 6% with placebo + MTX, and 18% with ADA (40 mg, biweekly) + MTX (both p ≤ 0.001) [13]. Prof. Burmester: "You don't see a big difference whether UPA is combined with MTX or not. If a patient is already receiving MTX under JAKi and tolerates it well, MTX can also be continued." "Often, patients decide to switch to monotherapy themselves," adds Prof. Rubbert-Roth.
How effective is UPA in PsA and AS patients with inadequate response to biologics?
The efficacy of UPA (15 mg, 1x daily) in PsA was evaluated in two randomized, phase III trials [14, 15]. SELECT PsA 1 included biologic-naive patients and SELECT PsA 2 included patients with inadequate response to biologics. In both studies, UPA showed significantly better response in terms of minimal disease activity (MDA) compared with placebo at 24 weeks (Figure 2). “The difference from placebo is comparable in both studies – this suggests good efficacy of UPA in PsA patients who have not responded to biologics,” Prof. Rubbert-Roth concluded. Similarly, for AS (Figure 2) [16, 17], “Again, the efficacy of UPA in terms of disease inactivity(Ankylosing Spondylitis Disease Activity Score-Inactive Disease, ASDAS-ID) in the two placebo-controlled, randomized, phase III trials SELECT AXIS 1 with biologic-naïve patients and SELECT AXIS 2 with patients with inadequate response to biologics comparable – perhaps marginally better in the biologic-naïve patients.” For Prof. Burmester, UPA “offers a good alternative to TNF and IL-17 inhibitors precisely because of its advantageous safety profile, which is readily accepted by patients.”
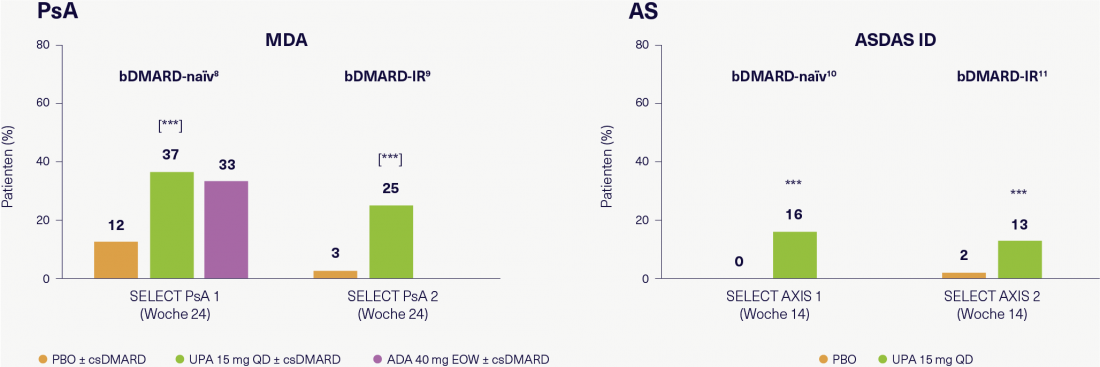
Figure 2: Efficacy of UPA in biologic-naive patients and patients with inadequate response to biologics in PsA and AS clinical trials. Adapted according to [14-17].
*** p ≤ 0.001 vs. PBO; [] = comparisons adjusted for multiplicity. All analyses were performed with NRI (non-responder imputation). ADA, adalimumab; AS, ankylosing spondylitis; ASDAS, ankylosing spondylitis disease activity score; bDMARD, biologic disease-modifying antirheumatic drug; csDMARD, conventional synthetic DMARD; EOW, biweekly; ID, inactive disease; IR, inadequate response; MDA, minimal disease activity; PBO, placebo; PsA, psoriatic arthritis; QD, once daily; UPA, upadacitinib.
How does UPA perform in practice compared to clinical trials?
On the final question of the performance of UPA under real-world conditions, Prof. Burmester presents the interim results of the post-marketing observational study UPwArds, which investigates the efficacy and safety of UPA as monotherapy or in combination with MTX in patients with moderate to severe RA in clinical practice in Germany [18]. After an observation period of 6 months, 65% of patients had achieved a DAS28-CRP value <2.6, and improvements in pain and fatigue were also observed. "Thus, we have data which are in good agreement with the results from clinical studies," the expert concluded.
Click here for the interview with Prof. Dr. med. Gerd Burmester on the topic of JAK inhibitors!
References
The references can be requested by professionals at medinfo.ch@abbvie.com.
Report and interview: Dr. sc. nat. Jennifer Keim
To the brief technical information of RINVOQ®.
This article was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, Cham.
CH-RNQR-220089_10/2022
Article online since 17.11.2022












