The three basic pillars of therapy in patients with atrial fibrillation (AF) are reduction of stroke risk, prevention of tachycardiomyopathy, and improvement of quality of life through symptom relief. Catheter ablation of atrial fibrillation is superior to antiarrhythmic therapy in terms of rhythm control and quality of life [1,2]. Current guidelines allow relatively much latitude in the indication for pulmonary vein isolation. Patients without structural heart disease with symptomatic paroxysmal AF or AF persistent for less than one year have the best chance of success. The earlier in the course of the disease the treatment is given, the higher the success rates of catheter ablation. Thanks to the great technological advances, the complication rates in experienced centers are very low, so that one can speak of a very safe procedure.
With a prevalence of 1.5-2%, atrial fibrillation is one of the most common cardiac arrhythmias in clinical practice. Prevalence increases with age. Among 80-year-olds, more than 8% are affected [3,4], men slightly more often than women. Idiopathic atrial fibrillation not associated with structural cardiopathy is reported to have a prevalence of up to 30% [5].
Depending on duration, atrial fibrillation is divided into paroxysmal (≤48 hours), persistent (>7 days), long persistent (>1 year), and permanent atrial fibrillation. In the latter, the arrhythmia is accepted and rhythm control is no longer sought. Similar to the NYHA score, arrhythmia burden is assigned to a symptom score (EHRA I-IV).
The mortality of patients with atrial fibrillation is increased by a factor of about 1.5 in men and 1.9 in women. This was the finding of analyses from the Framingham study. On the one hand, there is an increased risk of heart failure with a threefold increased risk of cardiac decompensation; on the other hand, there is a fivefold increased risk of cerebrovascular insult or systemic embolism. Using the CHA2DS2-VASc score, the risk of stroke can be estimated individually and somewhat more accurately.
Treatment options and indication for rhythm control
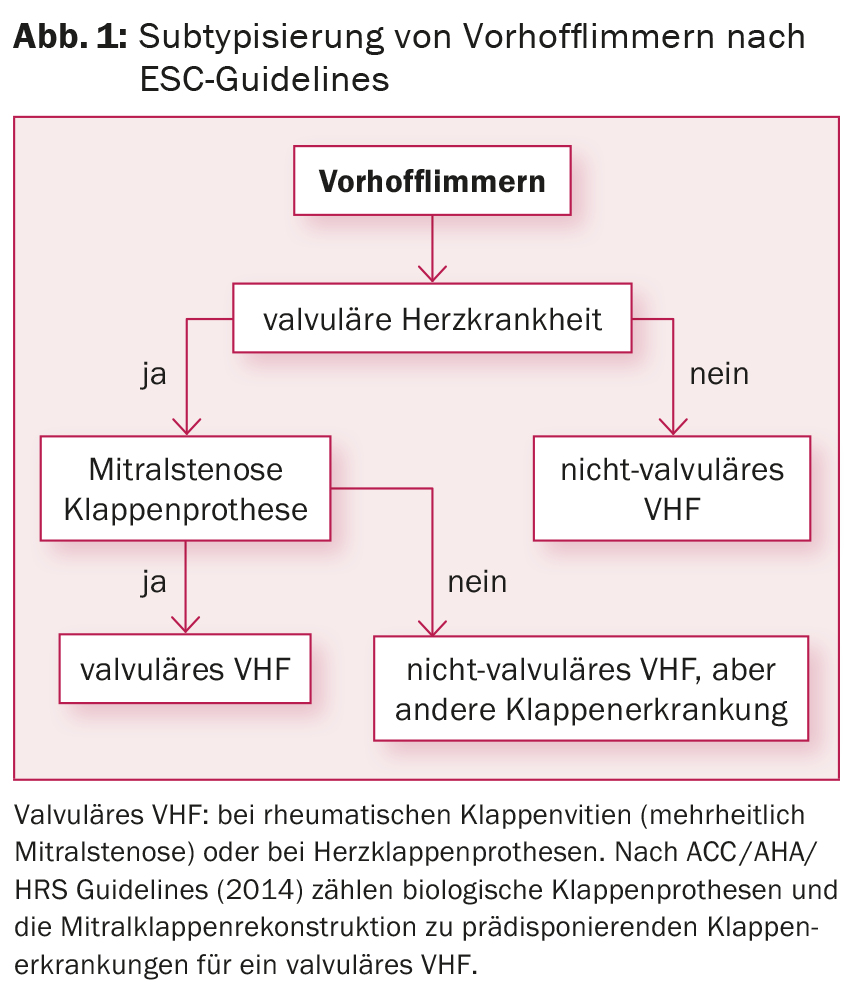
Taking into account risk stratification by CHA2DS2-VASc score, oral anticoagulation (OAC) with Marcoumar® or, in the case of nonvalvular AF ( Fig. 1), with one of the new anticoagulants (so-called direct oral anticoagulants [DOAK], e.g., rivaroxaban, dabigatran, apixaban, edoxaban) are established. To reduce symptoms and prevent tachycardiomyopathy (at sustained rates >120 bpm), frequency control should also be sought. Previous studies have shown no differences in the comparison of frequency control vs. rhythm control with regard to reduction of morbidity and mortality [6]. If medical therapy is not successful, AV nodal ablation with permanent pacemaker insertion is a possible option for rate control, especially in the presence of comorbidities.
Rhythm control has a definitive role in paroxysmal and persistent AF, especially in symptomatic patients despite strict rate control. Two basic procedures can be used: continuous drug therapy with antiarrhythmic drugs and the invasive approach using ablation procedures. In the acute situation with hemodynamic instability, this can also be achieved by electroconversion (ECV). If rhythm-regulating treatment is preferred over rate control, it should be initiated as close to the time of diagnosis as possible, because preservation of sinus rhythm becomes more difficult with increasing duration of AF [7,8].
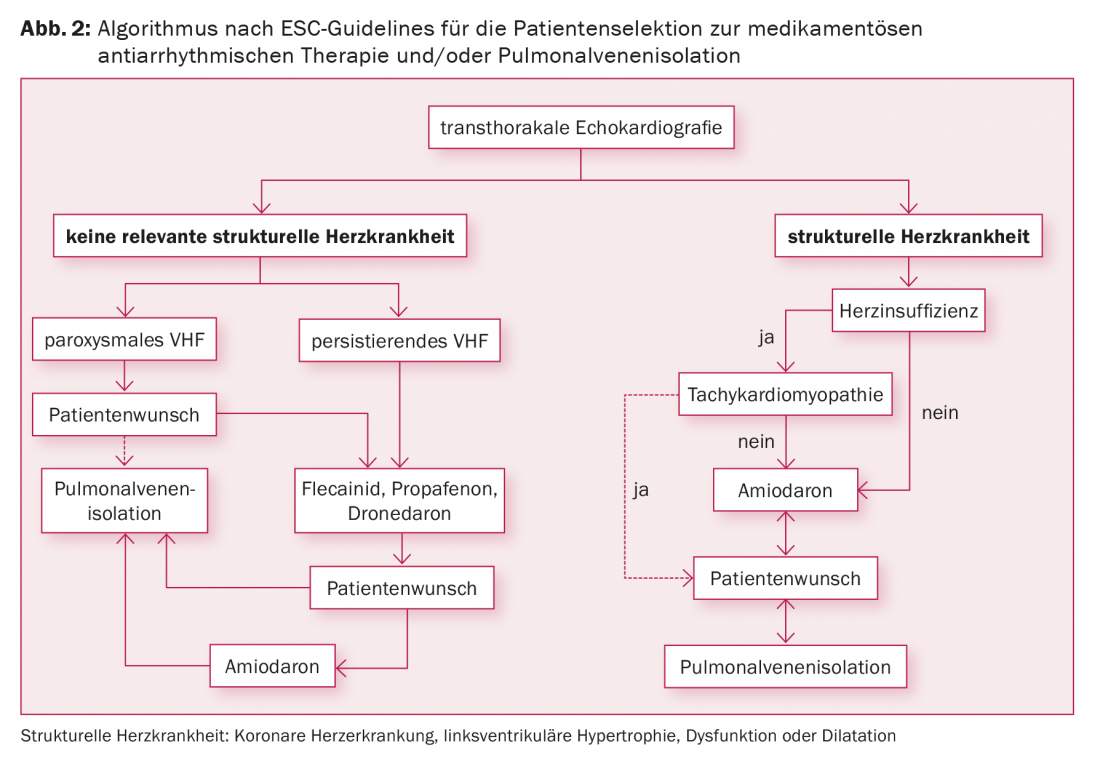
Indications for pulmonary vein isolation include symptomatic AF despite antiarrhythmic therapy, symptomatic AF and the patient’s desire not to have to take antiarrhythmic drugs, and symptomatic AF in combination with contraindications to antiarrhythmic drugs (Fig. 2) . Data showing prevention of cardioembolic events by catheter ablation are currently lacking. Therefore, according to current studies, the desire to stop OAK is not an indication for pulmonary vein isolation, because OAK must be continued even after successful ablation, depending on the CHA2DS2-VASc score.
Pulmonary vein isolation
Since the late 1990s, it has been known that more than 90% of the ectopic foci (so-called triggers) for the development of AF are localized in the pulmonary veins. Initial attempts were made to eliminate these active foci by direct local ablation. However, this not infrequently resulted in pulmonary vein stenosis. Today, it is known that isolation of the veins in the region where they join the left atrium (antrum) is sufficient and gentler. On the basis of these findings, pulmonary vein isolation has become established as an invasive therapeutic strategy through successful further development of various ablation and mapping techniques with consecutive improvement in terms of effectiveness and safety over the past 10-15 years.
Patients usually enter the hospital the day before the examination. On the same day, a transesophageal echocardiography is performed and, if necessary, a computed tomography scan or an MRI of the heart. These images can facilitate three-dimensional reconstruction of the left atrium.
Under analgesia (general anesthesia is not mandatory), several catheters are inserted via a venous access in the groin into the right atrium via the inferior vena cava and finally placed in the left atrium via a transseptal puncture (Fig. 3) .
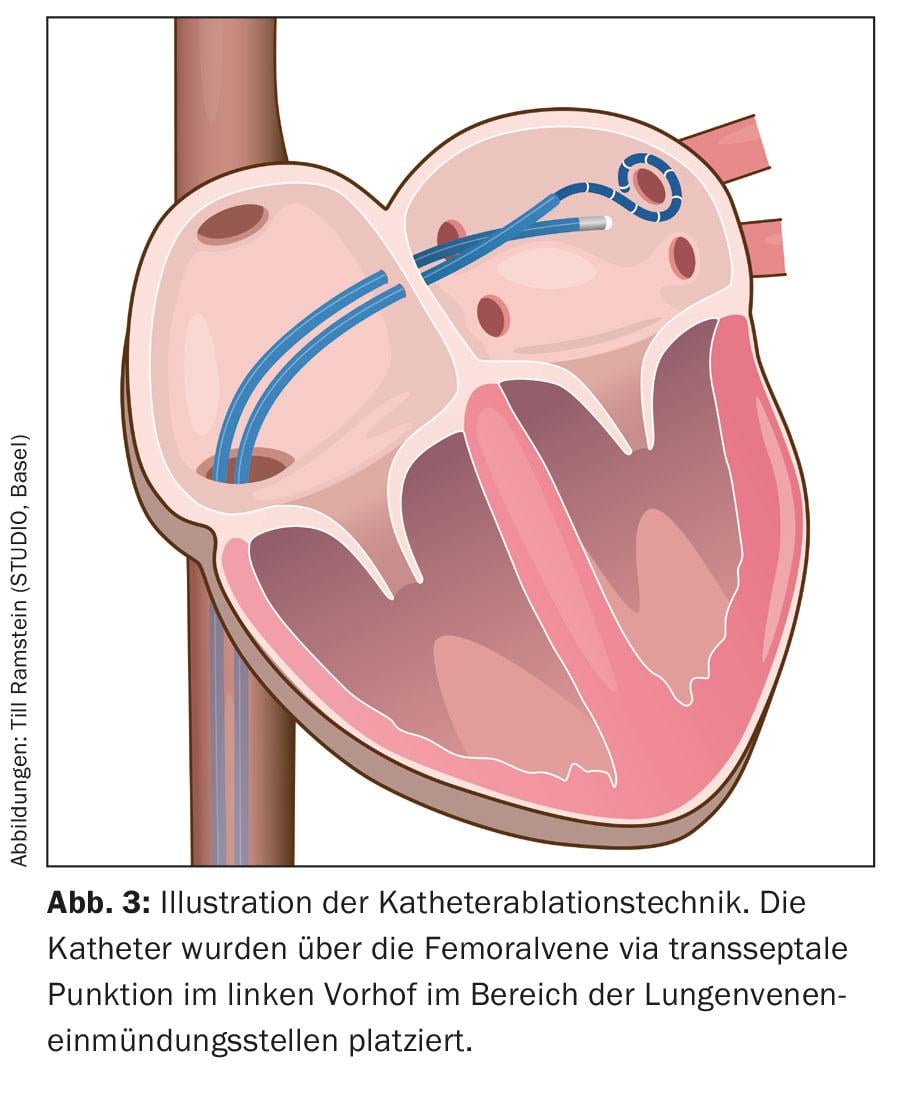
These catheters are used for a three-dimensional reconstruction of the ablation area, the so-called 3D mapping (Fig. 4). In addition to X-ray-based methods using pure mapping catheters to visualize the connection between the pulmonary veins and the left atrium, so-called “electroanatomical mapping methods”, which use a three-dimensional and not primarily X-ray-based system, have been integrated into the procedure.
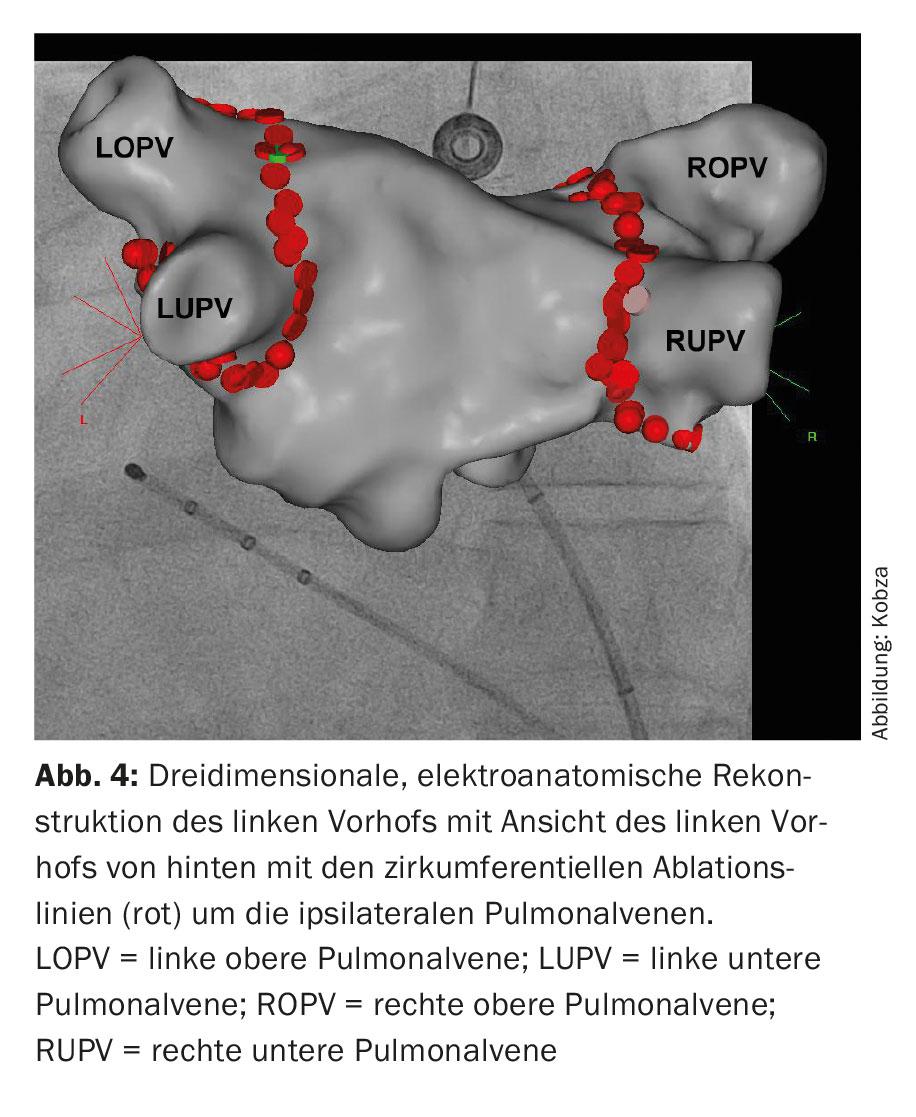
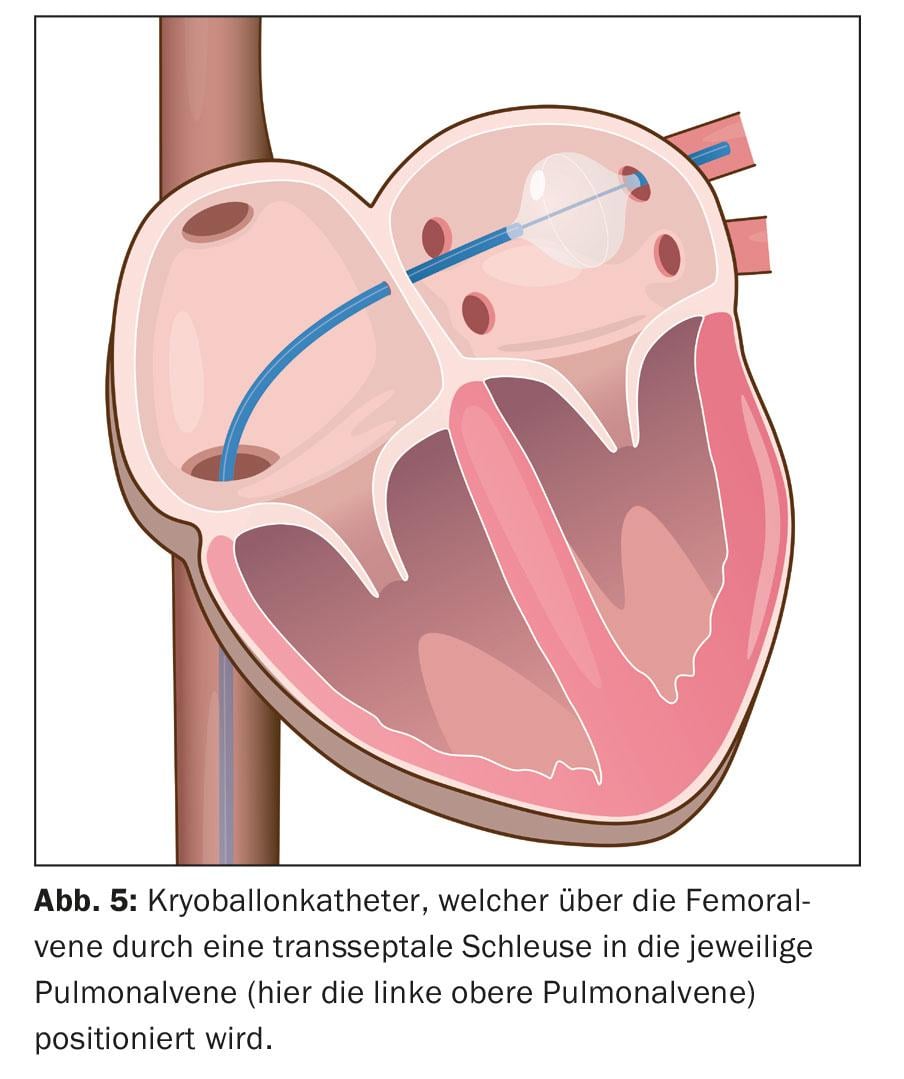
Image integration of CT- and MRI-based reconstructed left atrial anatomy is possible here. Subsequently, the ablation is performed. This involves circumferential point-to-point ablation in pairs around the ipsilateral pulmonary vein ostia using radiofrequency energy, a high-frequency alternating current (“wide area circumferential radiofrequency catheter ablation,” WACA) (fig. 4). Alternatively, isolation is achieved by icing, so-called cryoballoon ablation (Fig. 5). Novel circular and multipolar ablation catheters are also used. Laser procedures, which are also performed via a balloon-assisted catheter, are also becoming increasingly common. However, the potential asymmetry and size variability of pulmonary vein orifices and their anatomy variations may complicate the one-size-fits-all design of balloon-assisted ablation catheters. Radiofrequency ablation remains the most widely used. A circular mapping catheter is used to control the electrical isolation achieved. Isolation of all four pulmonary veins is the fundamental principle and primary goal of the procedure, otherwise recurrences of AF may occur, leading to treatment failure.
The intervention usually lasts 90-180 minutes. After removal of all catheters, a pressure dressing is applied. In most cases, patients can be discharged the following day.
OAK is not discontinued periinterventionally because this strategy is associated with fewer complications [9]. Postintervention, OAK is continued for at least three months (due to scarring in the ablation area) and then adjusted according to individual risk as defined by the CHA2DS2-VASc score.
Treatment success
An optimal method as a gold standard for the diagnosis of possible recurrences of atrial fibrillation has not yet been established. Depending on the center, long-term ECG checks (24 hours to 7 days) are performed postintervention at different intervals, usually after three, six, and twelve months, and then annually.
Success depends to a large extent on the experience of the center performing the procedure. Within the first three months, cardiac arrhythmias (atrial extrasystoles and atrial fibrillation) are sometimes still observed, which are not to be considered as recurrence (“blanking period”). If there is a continuous sinus rhythm afterwards, the procedure can be considered successful. The success rate is 80-90% in patients with paroxysmal atrial fibrillation without structural heart disease, with 20-30% requiring a repeat procedure. In persistent atrial fibrillation or the presence of structural heart disease, the success rate is 60-70%. Thus, ablation therapy has become very important, since even the most effective antiarrhythmic drug, amiodarone, achieves long-term rhythm maintenance of about 40-50%.
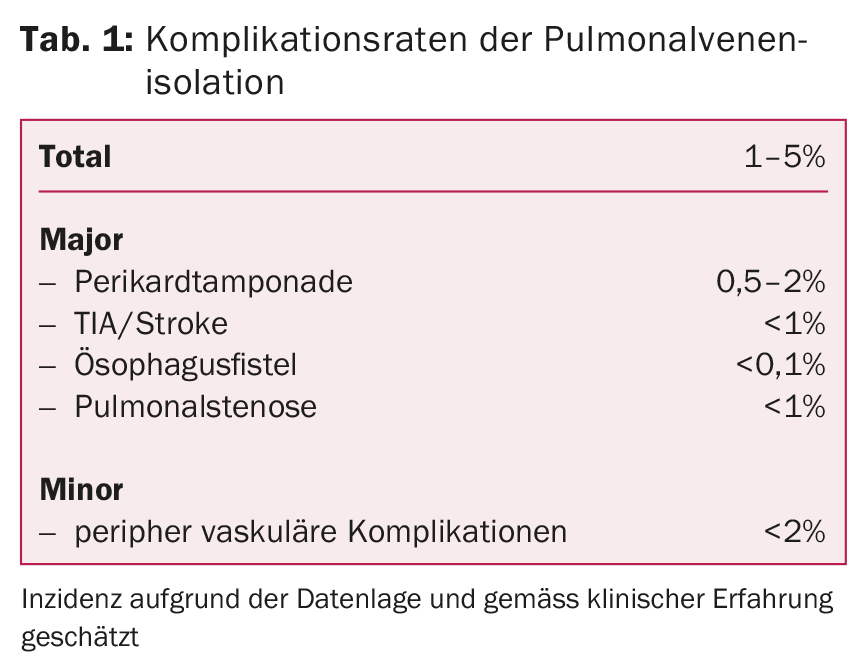
In experienced centers, this treatment is very safe and associated with few complications. The most dangerous complications include thromboembolic events (TIA, stroke), pericardial tamponade, pulmonary vein stenosis, or esophagoatrial fistulae. More frequently, complications may occur in the area of the injection site, such as secondary bleeding, hematomas or vascular injuries (Tab. 1).
Acknowledgements: A big thank you goes to Till Ramstein (STUDIO, Basel) for preparing figures 3 and 5.
Literature:
- Cosedis Nielsen J, et al: Radiofrequency Ablation as Initial Therapy in Paroxysmal Atrial Fibrillation. N Engl J Med 2012; 367(17): 1587-1595.
- Wazni OM, et al: Radiofrequency ablation vs antiarrhythmic drugs as firstline treatment of symptomatic atrial fibrillation: A randomized trial. JAMA 2005; 293: 2634-2640.
- Feinberg WM, et al: Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995; 155: 469-473.
- Heeringa J, et al: Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006; 27: 949-953.
- Sankaranarayanan R, et al: Comparison of Atrial Fibrillation in the Young versus That in the Elderly: A Review. Cardiol Res Pract 2013; 2013: 976976.
- The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators: A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347: 1825-1833.
- Cosio FG, et al: Delayed rhythtm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time or the first detected episode. Europace 2008; 10: 21-27.
- Kirchhof P: Can we improve outcomes in atrial fibrillation patients by early therapy? BMC Med 2009; 7: 72.
- Cappato R, et al: Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015 Jul 21; 36(28): 1805-1811.
CARDIOVASC 2015; 14(5): 3-6