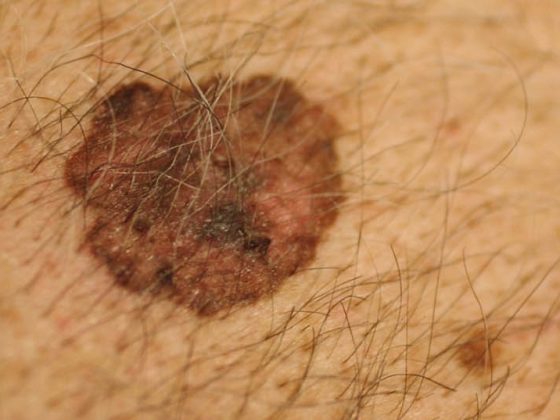
The incidence of white skin cancer is three to five times higher in HIV-infected individuals. Squamous cell and basal cell carcinomas occur at younger ages in HIV-infected individuals, are more likely to recur, and are more likely to cause death. Melanoma also has a slightly increased incidence in HIV-infected individuals. Moreover, there is a significantly worse prognosis compared to the normal population. While Kaposi’s sarcoma, non-Hodgkin’s lymphoma, and cervical cancer are significantly less common under HAART, the incidence for most non-AIDS-defining cancers has increased. In addition to liver cancer, Hodgkin’s lymphoma and anal carcinoma in particular show a strong increase under HAART. White skin cancer and melanoma also show an increase, albeit weaker. One reason why many tumors increase rather than decrease over the course of HAART may be that HIV patients are often diagnosed young, live longer on HAART, and “grow” into the higher risk group for tumors.
It has long been known that immunocompromised patients, especially organ transplanted patients, develop skin cancer significantly more frequently than immunocompetent patients. Thus, in this population, spinalioma is 65 times more common, basal cell carcinoma ten times more common, and melanoma three times more common. The risk of skin cancer is also increased in HIV-infected persons, although not to the same extent.
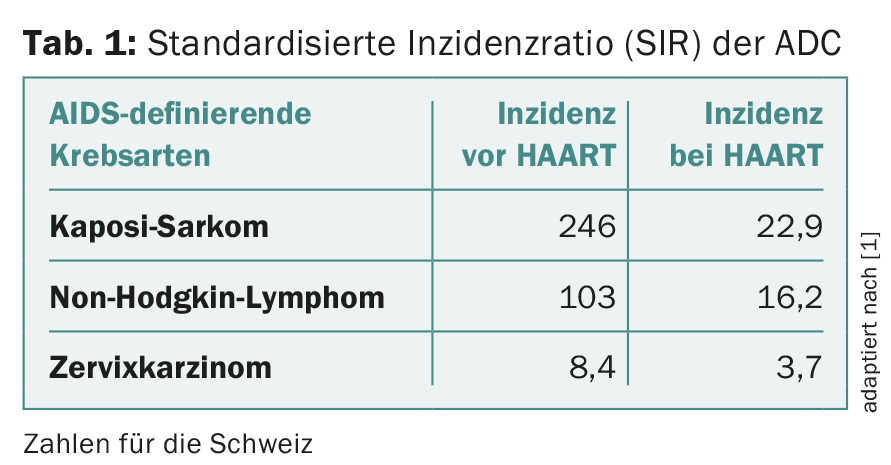
It is interesting to see how the incidence of the different cancers has changed with HAART (“highly active antiretroviral therapy”). Since the introduction of HAART, HIV-positive patients have a significantly better survival prognosis than before. Similarly, AIDS-defining diseases have decreased massively, so that we see them much less frequently in daily practice. AIDS-defining diseases also include malignant tumors (“AIDS-defining cancers”, ADC). These include Kaposi’s sarcoma, cervical carcinoma, and non-Hodgkin’s lymphoma (Table 1).
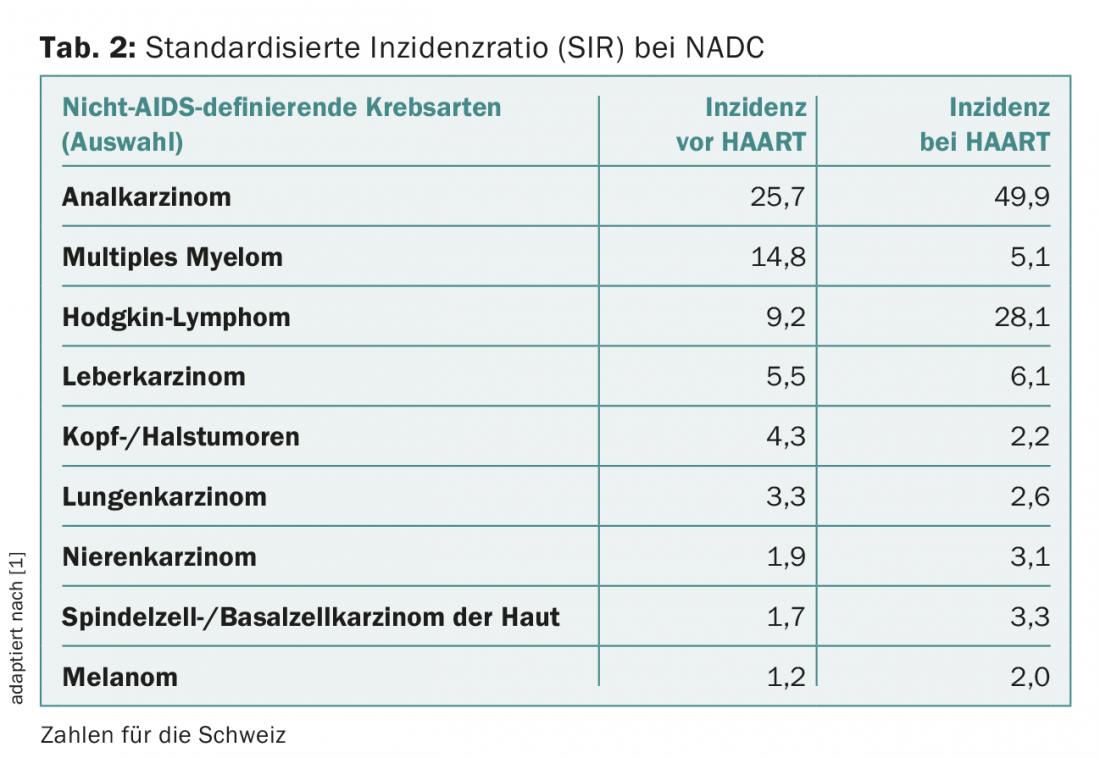
However, a number of other tumors have been described that occur more frequently in HIV-infected persons, the so-called non-AIDS-defining cancers (NADC) (Tab. 2) . These include non-melanoma skin cancer (NMSC). By this we mean squamous cell carcinoma of the skin (spinalioma, spindle cell carcinoma) and basal cell carcinoma (basal cell carcinoma). Bowen’s disease (entire epidermis) and actinic keratoses (basal cell layer only) are referred to as carcinoma in situ. Other cutaneous malignant tumors include the very rare Merkel cell carcinoma and melanoma.
This report focuses on the incidence and change in AIDS-defining and non-AIDS-defining cancers before and after the HAART era.
Facts
Overall, tumors have decreased significantly with HAART therapy, but on average there is still a 1.5- to 2-fold increased risk compared with the normal population.In a French study, the following cancers were diagnosed in HIV-infected individuals in descending frequency: Non-Hodgkin’s lymphoma 21.5%, Kaposi’s sarcoma 16.0%, lung cancer 9.4%, anal cancer 8.2%, Hodgkin’s lymphoma 7.6%, NMSC 6.8%, and liver cancer 5.6%. Non-AIDS-defining cancers accounted for a total of 68% of all tumors.
White skin cancer (NMSC)
The incidence of NMSC is increased three to five times in HIV-infected individuals. The ratio between basal cell and squamous cell carcinoma is 1:7. This is in marked contrast to kidney transplant recipients, where the ratio is reversed at 1.8:1. Risk factors for the development of NMSC are the same as in the normal population, first and foremost UV exposure. The extent to which human papillomavirus (HPV) contributes to the development of squamous cell carcinoma in HIV-infected individuals is unclear. NMSC clearly occur at younger ages in HIV-infected individuals, are more prone to recurrence, and are more likely to result in death. The consequence of this should be an aggressive treatment of precancerous lesions in this collective.
Melanoma
Melanoma has a slightly increased incidence in HIV-infected individuals. Depending on the source, the standardized incidence ratio (SIR) ranges from 1.1 to 2.6. It is discussed whether this slightly increased incidence may be related to an increased vigilance of patients towards skin lesions or to a more intensive medical control of these patients. However, it can be stated that HIV-infected patients with melanoma have a significantly worse prognosis than melanoma patients in the normal population. The lower the CD4 cell count, the worse the prognosis. However, Breslow penetration depth, the most important prognostic marker, is independent of CD4 cell count.
Kaposi’s Sarcoma
Kaposi’s sarcoma is one of the AIDS-defining tumors. It is a vascular proliferation. Before the HAART era, Kaposi’s sarcoma usually resulted in death, with a median survival of 18 months, as HIV-associated Kaposi’s sarcoma is an aggressive variant. Under HAART, the incidence could be massively reduced (Tab. 1) . Kaposi’s sarcoma can affect not only the skin but also internal organs. The human herpes virus 8 (HHV8) is responsible. Therapy consists primarily of immune reconstitution.
Anal carcinoma
Anal carcinoma is associated with HPV. The incidence is increased especially in homosexual men. Risk factors include a low CD4 cell count, infection with high-risk HPV types (HPV-16, -18, -31, and -33), and infection with multiple HPV types. The pathogenesis is comparable to the pathogenesis of cervical carcinoma. HIV-infected individuals, regardless of sexual practices, have a two- to six-fold increase in HPV colonization in the anus compared with HIV-negative individuals. Homosexual men with HIV have anal HPV in 93% of cases, compared with 61% of non-HIV-infected homosexual men.
Cutaneous lymphomas
Cutaneous lymphomas play a minor role due to their rarity, but should be mentioned here for the sake of completeness. Possible types include CD30+ large cell cutaneous T-cell lymphoma, mycosis fungoides and Sézary syndrome, and cutaneous forms of Hodgkin’s lymphoma.
ADC and NADC in the course under HAART
HAART was introduced in 1996 and has had a major impact on the development of various malignant tumors in HIV-infected individuals. Thus, under HAART, the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma occur massively and cervical carcinoma occurs significantly less frequently. In the Swiss HIV Cohort Study, which has been collecting prospective data on HIV-infected persons for more than 20 years, the SIR for all AIDS-defining cancers dropped from 136 to 14.7.
In contrast, the SIR for most non-AIDS-defining cancers has increased. In addition to liver cancer, Hodgkin’s lymphoma and anal carcinoma in particular show a strong increase under HAART. Increases were also seen in white skin cancer, with an SIR before HAART of 1.7 and under HAART of 3.3. Melanoma shows a weaker increase, from 1.2 to 2.0. However, some non-AIDS-defining cancers also experienced a decrease with HAART, such as multiple myeloma (from 14.8 to 5.1) and head and neck tumors (from 4.3 to 2.2) (Table 2).
It is interesting to compare the frequency of HPV-associated tumors between organ transplant recipients and HIV-infected individuals. In the field of skin tumors, there is a marked increase in NMSC among organ transplant recipients with an SIR of 28. Among HIV-infected individuals, there is only an SIR of 4 A similar relationship is shown by melanomas, the incidence of which is hardly increased in HIV-infected persons, but is doubled to tripled in organ transplant patients. In the case of anal carcinoma, the ratios are reversed: here, HIV-infected patients show an SIR of 28, and organ transplant patients of 5 In addition, other virus-induced tumors that are due to infections with HHV8, Epstein-Barr virus (EBV) or hepatitis B- resp. C viruses, more frequently in HIV-infected individuals than in organ transplant recipients.
Discussion
The exact reason for the partially increased cancer risk in HIV-infected persons is ultimately not clear. It is known that CD4 lymphopenias generally increase the risk of cancer. Under HAART, patients live significantly longer even with low CD4 cell counts. In this context, it is interesting to note that the duration of HIV disease – but not the severity – increases the risk of cancer. Moreover, CD4 and CD8 cell dysfunction occurs, leading to a decrease in immunological tumor surveillance. Oncogenic viruses such as HHV8, HPV, EBV, or human T-cell lymphotropic virus (HTLV1/2) can proliferate more easily. In addition, there is a predominance of Th2 cytokines under HIV infection. This has a consecutive effect on angiogenesis, apoptosis, antigen presentation, immune evasion and transcription of various oncogenes.
Also, certain HIV proteins play a role in the development of some malignant tumors. Thus, HIV can have direct effects at the cellular or even gene level. HIV can activate protooncogenes, interfere with the cell cycle, inactivate tumor suppressor genes (e.g., p53), and contribute to gene destabilization or alteration.
Why do many tumors increase and not decrease under HAART?
Interestingly, NADC are only increasing among Caucasians, not Black Africans or other ethnic groups. However, there may be a bias here, as the quality of cancer reports could vary. Further, it should be noted that this increase is seen only in HIV-infected men, not in HIV-infected women. As in the normal population, the greatest risk factor is age. The fact that the majority of HIV-infected patients are young, that they live significantly longer with HAART and thus “grow into” a higher risk group, makes it understandable that certain cancers, the majority of which occur in old age, increase during the course of HAART, or despite HAART. Especially for NMSC such as spinalioma, this is probably the determining factor, this due to the increasing cumulative UV dose at older ages. No association was found between histologically unfavorable factors (in either basal cell carcinoma or spinalioma) and CD4 cell count, HIV viremia, HAART duration, duration of HIV disease, or CD4 nadir.
It is also speculated that HIV-infected individuals have a higher risk tolerance and possibly a different (unhealthier) lifestyle. The extent to which the actual therapy has a negative influence has not yet been conclusively clarified. Some HIV medications are at least photosensitizing.
Outlook
Screening programs have been proposed for some ADC and NADC (anal carcinoma, cervical carcinoma, breast cancer, colorectal carcinoma, liver carcinoma, and prostate carcinoma). To date, there is no recommendation for skin tumor screening. Although skin tumors are not as common in HIV-infected individuals, they do occur at a younger age, which, by analogy with organ transplant recipients, would warrant a screening program.
Literature:
- Franceschi S, et al: Br J Canc 2010; 103: 416-422.
Further reading:
- Wilkins K, et al: J Am Acad Dermatol 2006; 54: 189-206.
- Deeken JF, et al: CID 2012; 55(9): 1228-1235.
- Grulich AE, et al: Lancet 2007; 370: 59-67.
- Silverberg MJ, et al: J Natl Cancer Inst 2013; 105: 350-360.
- Guiguet M, et al: Lancet Oncol 2009; 10: 1152-1159.
- Lanoy E, et al: Int J Cancer 2011; 129: 467-475.
- Schulz TF: Int J Cancer 2009; 125(8): 1755-1763.
- Garlassi E, et al: J Acquir Immune Defic Syndr 2012; 60(2): e63-65.
- Hofbauer GFL, et al: Swiss Med Wkly 2009; 139(29-30): 407-415.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(11-12): 25-27.
DERMATOLOGIE PRAXIS 2016; 26(5): 12-14