Modulation of S1P receptors is an important and interesting therapeutic target in the treatment management of multiple sclerosis due to their functional multifold. Currently, three S1P receptor modulators are available for RRMS patients and one for SPMS patients. None of the S1P receptor modulators showed a negative effect on cognition and fatigue.
Multiple sclerosis (MS) is a common neurological disease that results in demyelination and axonal damage due to immune-mediated chronic inflammation of the central nervous system (CNS) [1]. The majority of patients (approx. 85%) initially experience a relapsing-remitting course (RRMS), which in the course of the disease develops into a secondary chronic progressive phase [2]. RRMS is defined by relapses with new or increasing clinical symptoms. This is followed by phases of partial or complete remission. During periods of remission, disease progression is not evident [1,2].
The diagnosis of MS requires objective evidence of an inflammatory event in the CNS and additional evidence of spatial and temporal spread of the disease process (dissemination in space and time). The main paraclinical tests used to confirm the diagnosis are magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) examination [3].
There is now a consensus that relapses alone are not informative with regard to the disease process and its development. Thus, the number of relapses provides little information about how much structural and functional damage is actually present. Moreover, the loss of brain volume, which can occur very early in the disease process, is not necessarily associated with thrust activity. From a pathological perspective, active tissue damage in MS is associated with inflammation. In addition, other mechanisms such as oxidative stress leading to mitochondrial damage are likely involved in the induction of demyelination and neurodegeneration in both relapsing and progressive phases of MS [4]. Because neither the RR nor the progressive phase of MS have separable pathological or mechanistic features, quantitative rather than qualitative differences between these phases appear to be responsible for which predominant phenotype results [4,5]. Today, modern MS therapy aims at early diagnosis and early use of course-modifying drugs in order to influence disability progression as favorably as possible at an early stage [1,2,6].
Fatigue and cognitive problems
However, it is not only the physical disability progression that poses major challenges to affected patients and treating physicians. In coping with everyday life, it is mainly symptoms such as fatigue and cognitive problems. It is estimated that 75-95% of all MS patients suffer from fatigue and 40-50% from cognitive performance decline [7]. Both symptoms have serious effects on social participation, occupational ability, and overall quality of life [7,8]. It is important to include the above symptoms in the assessment of progression and to record them regularly, once a year, with sensitive instruments, regardless of the EDSS [9]. This allows to assess the individual in question in the longitudinal course. In cognition, there is evidence that patients who already show cognitive abnormalities at diagnosis are at increased risk for aggressive pathology with severely disabling progression [10]. From this, a therapeutic algorithm better tailored to the individual case could be derived right at the beginning.
Since fatigue is the symptom which, in addition to cognitive disorders, is responsible for early retirement and disability, care must be taken when selecting immunotherapy to ensure that drugs are not used which are expected to exacerbate symptoms.
Course modifying therapies
Disease modifying treatment options are known as disease modifying therapies (DMTs) and can be administered as tablets, capsules, injections or infusions. The goal is to reduce both the annual relapse rate and the progression. Preparations with different modes of action are available. In this work, we focus on S1P receptor modulators. Four drugs of this class are currently approved in Switzerland – fingolimod, ozanimod and ponesimod for RRMS, siponimod as the only S1P receptor modulator for SPMS patients [11–14].
Sphingosine-1-phosphate receptors
Sphingosines are structural components of the cell membrane that give rise to many bioactive lipids, such as S1P. This is a multifunctional bioactive lipid metabolite that acts as a soluble signaling molecule and circulates in the blood and lymphatic system [15]. S1P is involved in a variety of physiological and pathophysiological processes through its interaction with S1P receptors, which are high-affinity G-protein-coupled cell surface receptors found throughout the body [16].
S1P has a binding affinity for five different S1P receptor subtypes through which cellular signals are transduced. S1P1, S1P2, and S1P3 receptors are ubiquitously expressed, with each subtype having different cell specificity. In contrast, S1P4 and S1P5 receptor expression is less widespread. S1P4 receptors are restricted to lymphoid and hematopoietic tissues, and S1P5 receptors are found in the spleen and CNS white matter, mainly in oligodendrocytes (Table 1) [17].
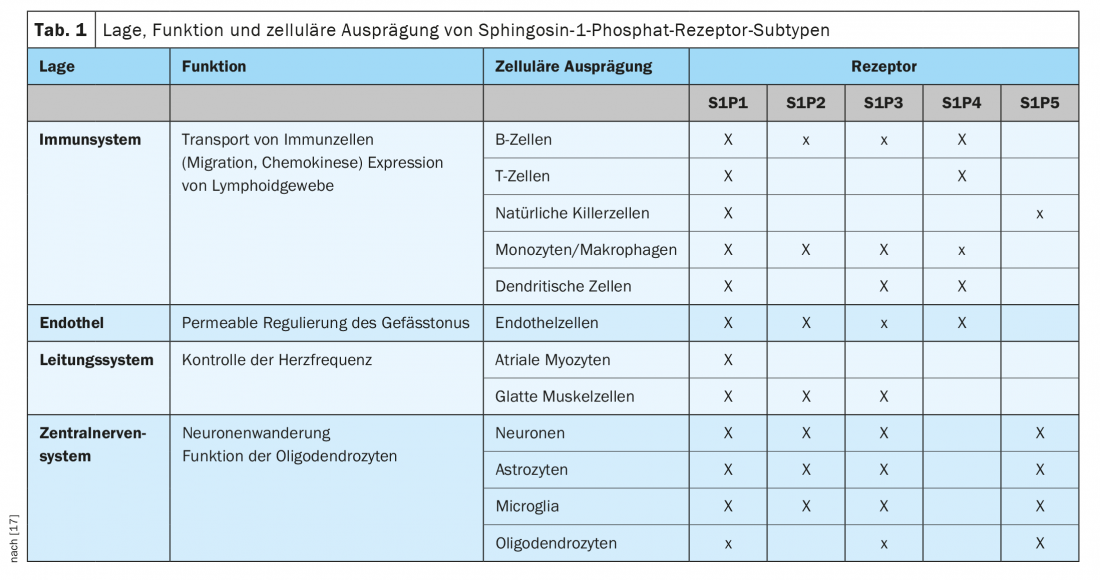
Accordingly, S1P receptors are involved in many biological functions, including cell proliferation, angiogenesis, cytoskeletal organization, endothelial cell chemotaxis, immune cell transport, and mitogenesis. In addition, they also play a role in brain and heart development, skin cell proliferation, vascular permeability, and regulation of vascular and bronchial tone [18]. This functional diversity makes modulation of S1P receptors an interesting and important therapeutic target.
The development of S1PR modulators
S1P receptor modulators can act as both functional antagonists and traditional agonists, depending on the S1P receptor subtype or subtypes targeted, and can have varying specificity [19]. These are small molecules for oral administration. The main immunological mechanism of S1P receptor modulators is based on the regulation of lymphocyte migration through interaction with the S1P1 receptor. This mechanism is based on a concentration gradient of S1P, which is physiologically higher in blood than in lymphoid tissues. When the ligand binds the receptor on cells expressing C-C motif chemokine receptor 7 (CCR7), the S1P1 receptor is internalized and degraded, resulting in a loss of responsiveness to the S1P gradient. Consequently, lymphocytes are retained in the thymus and secondary lymphoid organs, resulting in lower numbers of lymphocytes in the peripheral blood and limiting the migration of inflammatory cells [20].
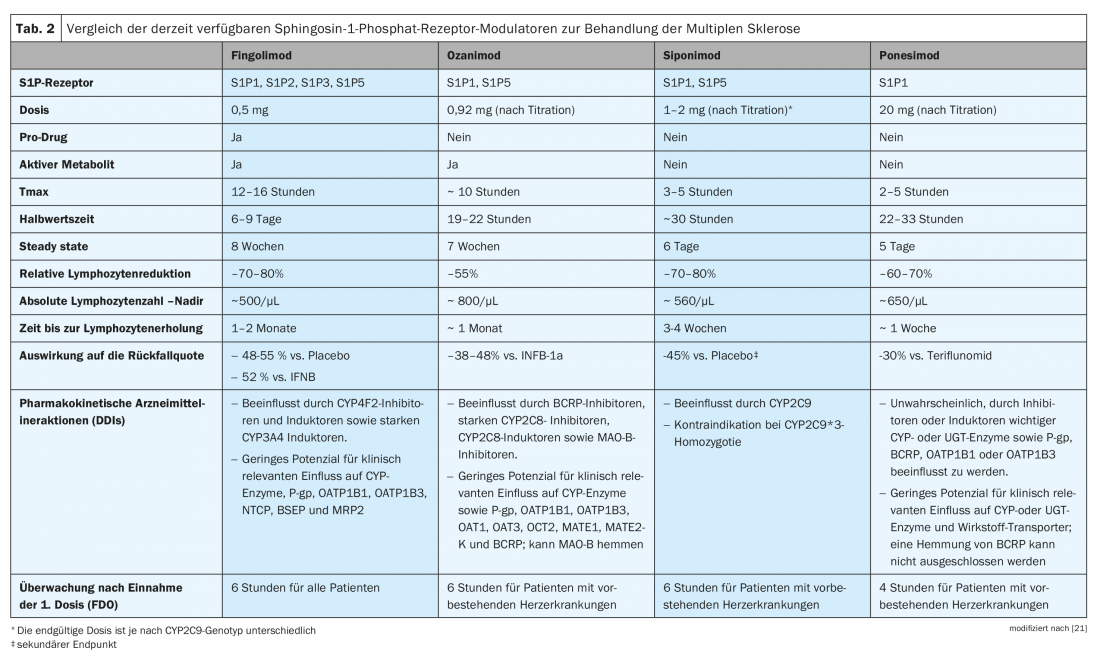
Some of the S1P receptor modulators act as prodrugs (fingolimod) that require phosphorylation or other metabolic steps to be converted to the pharmacologically active compound, while others are directly active drugs (siponimod, ozanimod, ponesimod). Therefore, despite a comparable mechanism of action, the S1PR modulators exhibit different pharmacokinetic properties (Table 2) [21]. In order to make a tailored therapy decision, it is therefore essential to consider the preparations of the active substance class in a differentiated manner.
S1PR modulators at a glance
Fingolimod (FTY) was the first S1PR modulator approved for RRMS therapy. The prodrug fingolimod hydrochloride is stereoselectively phosphorylated to the active metabolite (S)-fingolimod phosphate primarily in the liver by sphingosine kinase-2 (SphK-2). This is an analogue of S1P. Receptor modulation leads to downregulation of cell surface receptors and reduces sensitivity to endogenous ligand. It thus blocks the leakage of lymphocytes from the lymph nodes into the peripheral blood circulation and reduces the number of lymphocytes in the blood to 20-30% of the initial value. Because activation of S1PR3 can lead to an initial agonistic effect on cardiac cells, causing atrioventricular (AV) blocks, a comprehensive assessment should be performed before initiating therapy. The patient should be evaluated for bradycardia, long-QT, AV block, and other arrhythmia risk factors and monitored for six hours after the first administration. -In addition, pre-existing conditions should be excluded. Liver enzymes, blood count and blood pressure should be monitored constantly. If lymphocytes fall below 200/ml, a treatment break is indicated. Elevated liver enzyme levels could be addressed with a dose reduction to fingolimod every other day. However, this may result in reduced efficacy. Especially in younger patients and in patients switched from natalizumab, there is a risk of rebound effects with an increased risk of relapses and radiological activity [22,23].
Study data comparing fingolimod to placebo or interferon beta 1a demonstrated a relapse reduction of approximately 50% and a 74% reduction in the development of new or enlarging brain lesions at [24–26]. In addition, a favorable effect on cognitive performance, as measured by the PASAT-3, was demonstrated with fingolimod compared with placebo [27]. However, S1PR modulator was also detected to increase the risk of herpes virus and cryptococcal infec-tio-ns, with the risk increasing with longer treatment duration and older age [28]. Other potentially serious complications may include liver damage with transaminase elevation, skin cancers such as basal cell carcinoma, and eye complications (e.g., macular edema, retinal hemorrhage, and retinal vein occlusion).
Siponimod was approved in Switzerland in 2020 for the treatment of clinically isolated syndrome, relapsing-remitting multiple sclerosis, and active secondary progressive multiple sclerosis [29,30]. This S1PR inhibitor selectively binds to the S1PR1 and S1PR5 subtypes. The drug thus prevents the migration of lymphocytes from the lymph nodes and reduces the recirculation of T cells into the central nervous system (CNS) to limit inflammation in the CNS. The hypothesis behind this suspects fewer off-target effects than with fingolimod [30]. Analogous to fingolimod, pre-existing conditions such as ocular, cardiac, and severe liver disease must be checked and vaccination status updated (e.g., chickenpox). Other potential complications associated with induced immunosuppression include increased risk of infection and skin cancer. Possible side effects at the beginning of therapy (e.g. bradycardia, AV block) can be reduced with the help of a titration scheme. This provides for administration of 0.25 mg/day on days 1 and 2, followed by 0.5 mg on day 3, 0.75 mg on day 4, 1.25 mg on day 5, followed by a maintenance dose of 2 mg daily. It should be noted that siponimod is metabolized mainly by CYP2C9. A less common variant of this enzyme (CYP2C9*3) has reduced metabolizing activity. For this reason, heterozygotes for CYP2C9*3 should receive half the maintenance dose, whereas homozygosity is a contraindication to treatment with siponimod [32].
Compared with placebo, a reduction in the number of contrast-enhancing lesions in T1 weighting and new or newly enlarged T2 lesions was observed at 6 months (84% for siponimod 10 mg, 80% for siponimod 2 mg, and 58% for siponimod 0.5 mg) [30]. In addition, a significant reduction in disability progression in SPMS patients has also been demonstrated [29]. Younger sufferers in particular, those with a lower EDSS at baseline, shorter disease duration at baseline, and signs of acute inflammatory activity respond well to treatment [31].
Furthermore, it is worth mentioning that patients under therapy with siponimod showed a benefit on information processing speed measured with the SDMT. This showed not only a delay of cognitive deterioration but also a clinically relevant cogitative improvement within 6 months [33].
Ozanimod has also been approved for the treatment of RRMS and, similar to siponimod, selectively binds to S1PR1 and S1PR5 subtypes, preventing lymphocyte leakage from secondary lymphoid organs [34]. In patients without current/pre-existing cardiac symptoms, no initial dose observation is required when the drug is up-titrated (0.23 mg on days 1-4, 0.46 mg on days 5-7, then 0.92 mg once daily).
Study results show lower annualized relapse rates, fewer annualized gadolinium lesions, new or enlarging T2 lesions, and lower rates of brain atrophy with a favorable safety profile compared with interferon beta-1a [35]. However, disability progression was comparable between groups. Similar to siponimod, positive effects on cognitive speed (SDMT) were also found under ozanimod [36].
Ponesimod was approved in November 2020 for adults with active relapsing-remitting multiple sclerosis, making it the newest addition to this class of agents. The S1PR modulator with high affinity for S1PR1 has a short half-life of 32 hours [37]. After treatment is discontinued, lymphocyte counts normalize within seven days. Initial dose monitoring is required in patients with current or pre-existing cardiac disease/conditions, as initiation of treatment with Ponvory results in a transient reduction in heart rate and atrioventricular (AV) conduction abnormalities.
The efficacy of ponesimod has already been established in a dose-finding Phase II study and later confirmed in a larger Phase III RCT. Compared with placebo, treatment with ponesimod (10 mg, 20 mg, and 40 mg) met the primary endpoint of reducing the cumulative number of new gadolinium-enhancing lesions in a phase II study in RRMS patients [38]. Transient bradycardia and atrioventricular block occurred in 2% of participants each. Dyspnea or respiratory side effects were registered in a dose-dependent manner [38]. Ponesimod was also shown to be superior to an active comparator (teriflunomide) with respect to fatigue [39].
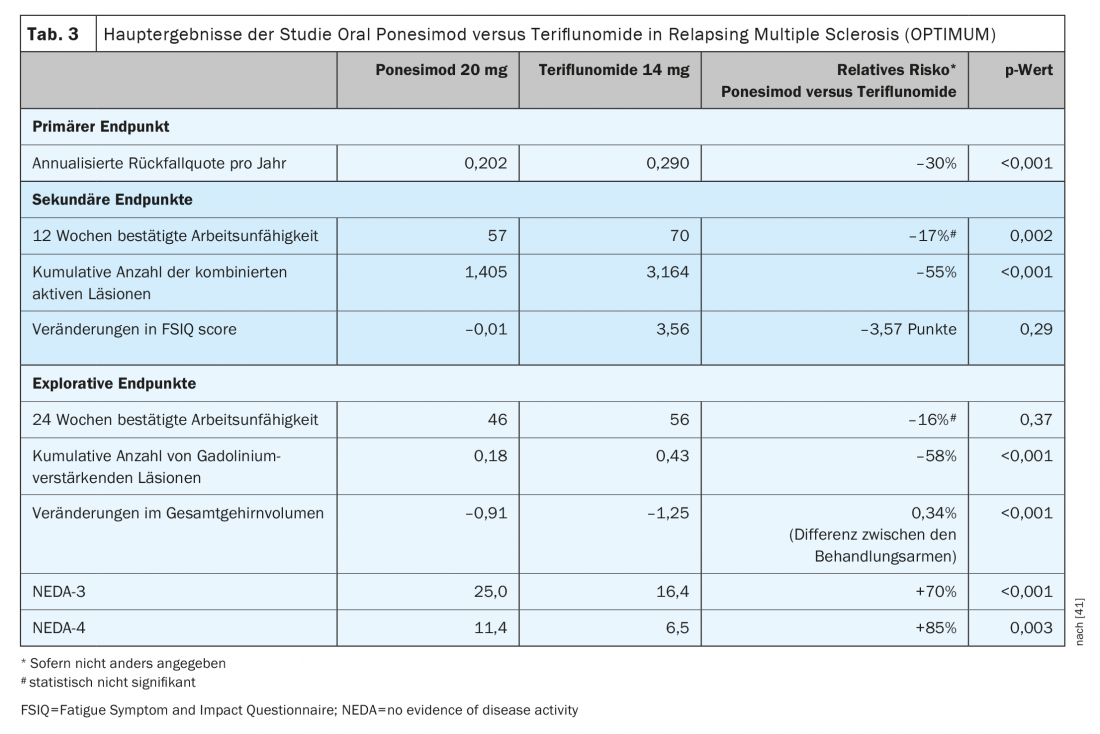
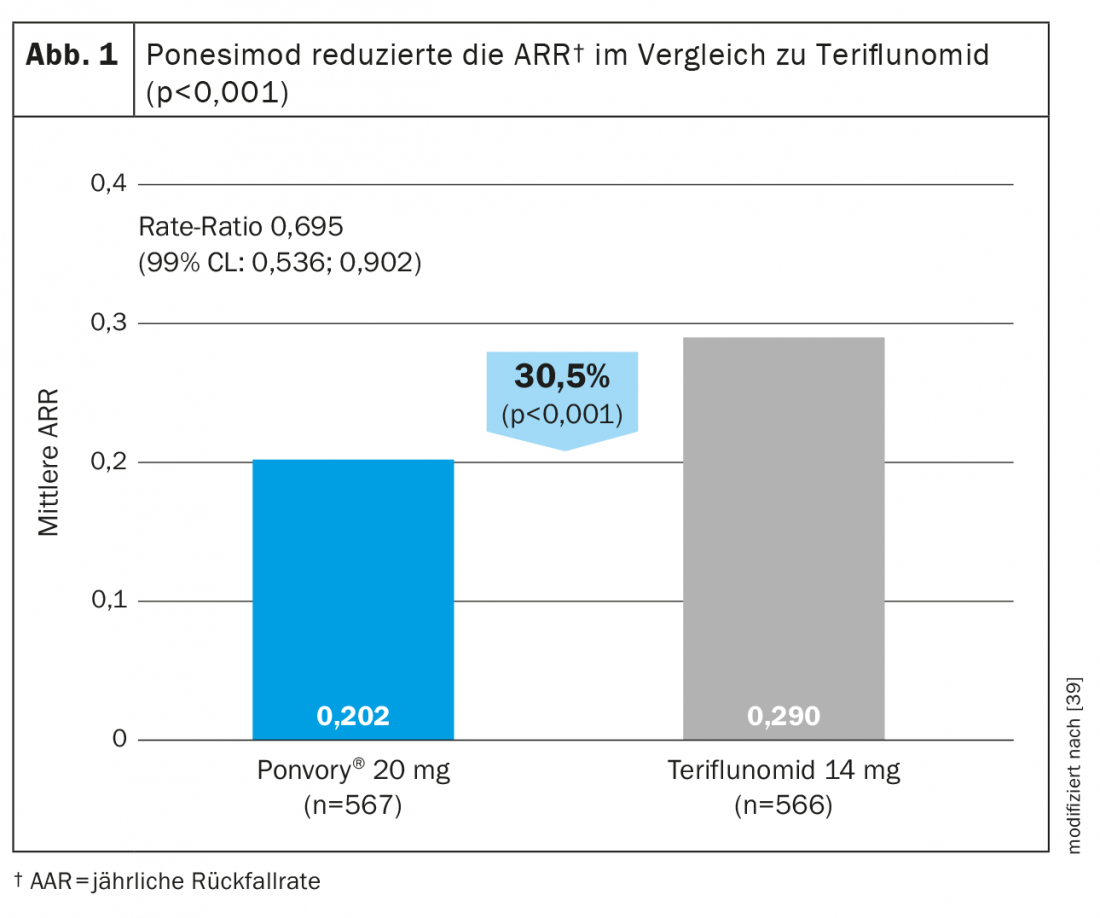
Ponesimod is the first approved oral DTM to be evaluated against another established oral agent for the treatment of relapsing-remitting multiple sclerosis. Ponesimod 20 mg was compared with teriflunomide in a phase III study trial (OPTIMUM) in patients with relapsing multiple sclerosis (Table 3) [39]. The primary endpoint was the annulized relapse rate over the study period. Secondary end points included the number of new gadolinium-enhancing T1 lesions and the number of new or enlarging hyperintense T2 lesions from baseline to week 108, and the time to 3-month confirmed disability progression. Exploratory endpoints included patients without relapse and mean percent change in brain volume from baseline to week 108.
The study showed that ponesimod achieved a 30.5% greater reduction in ARR compared with teriflunomide (Fig. 1) . In addition to magnetic resonance imaging activity and brain volume loss, fatigue was also better reduced with ponesimod compared to the active comparator teriflunomide [38]. In terms of reducing confirmed disability progression, efficacy was comparable in both test groups [39].
The proportion of patients with at least one treatment-emergent adverse event (TEAE) or serious adverse event (SAE) was also comparable in both treatment groups. The majority of TEAEs were mild to moderate and did not result in treatment discontinuation. The most common side effects in the ponesimod group were: ALT elevation, nasopharyngitis, headache, upper respiratory tract infection, and hypertension.The beneficial effect of ponesimod 20 mg on MS disease was maintained for about eight years, with no new side effects detected [40].
Overall, the treatment effects of ponesimod appear to be consistent with those of other S1P receptor modulators, with no unexpected safety concerns, yet this should be interpreted with caution in the absence of head-to-head comparative studies.
Unlike fingolimod, but similar to ozanimod and siponimod, the uptitration regimen of ponesimod minimizes the effect on heart rate and rhythm AES at the first dose, so prolonged cardiovascular monitoring is not required at baseline. In addition, the feature of a shorter half-life of ponesimod compared to fingolimod may be an advantage in case of vaccination or pregnancy and in the management of side effects [41].
Conclusion
The class of S1PR modulators offers a new effective and exciting option for MS treatment management. Even though the currently approved preparations are based on the same mechanism of action, they differ in their handling and profile. Therefore, in order to make an individually tailored therapy decision, information such as pharmacodynamics, pharmacokinetics, necessary measures before and at the start of therapy as well as during therapy, and possible drug interactions should be considered.
Take-Home Messages
- Modulation of S1P receptors is an important and interesting therapeutic target due to their functional diversity.
- Currently, three S1P receptor modulators are available for RRMS patients and one for SPMS patients.
- Fingolimod acts on S1P1, S1P2, S1P3 and S1P5.
- Siponimod, ozanimod and ponesimod act specifically on S1PR1 and S1PR5.
- In MS patients without current/pre-existing cardiac symptoms, no observation is required with initial administration of ozanimod and ponesimod.
- The shorter half-life of siponimod, ozanimod, and ponesimod helps lymphocytes recover more quickly after treatment is discontinued.
- The aforementioned S1P receptor modulators have not shown negative effects on cognition and fatigue.
Literature:
- Aktas O, et al: Diagnosis of multiple sclerosis: revision of the McDonald criteria 2017. Neurologist 2018; 89: 1344-1354.
- www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS (last accessed on 01.09.2022)
- Thompson AJ, Banwell BL, Barkhof F, et al: Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;1 7(2): 162-173.
- Lassmann H: Cortical lesions in multiple sclerosis: inflammation versus neurodegeneration. Brain. 2012; 135(Pt 10):2904-2905.
- Tobin WO, Kalinowska-Lyszczarz A, Weigand SD, et al: Clinical correlation of multiple sclerosis immunopathologic subtypes. Neurology. 2021; 97(19): e1906-e1913.
- Baecher-Allan C, et al: Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018; 97(4): 742-768.
- Penner IK: Evaluation of cognition and fatigue in multiple sclerosis: daily practice and future directions. Acta Neurologica Scandinavica 2016; 134 (Suppl. 200): 19-23.
- Kobelt, et al: New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017; 23(8): 1123-1136.
- Penner IK, Warnke C: Cognitive impairment in multiple sclerosis. DGNeurology 2021; 3: 184-186.
- Pitteri et al: Cognitive impairment predicts disability progression and cortical thinning in MS: An 8-year study. Mult Scler. 2017; 23(6):848-854.
- Gilenya® SmPC, as of November 2020. www.swissmedicinfo.ch
- Zeposia® SmPC, as of July 2020. www.swissmedicinfo.ch
- Ponvory® SmPC, as of November 2021. www.swissmedicinfo.ch
- Mayzent® Technical Information, as of October 2020. www.swissmedicinfo.ch
- Rosen H, Germana Sanna M, Gonzalez-Cabrera PJ, Roberts E: The organization of the sphingosine 1-phosphate signaling system. Curr Top Microbiol Immunol 2014; 378: 1-21.
- Kunkel GT, Maceyka M, Milstien S, Spiegel S: Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 2013; 12(9): 688-702.
- Cartier A, Hla T: Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science. 2019;366(6463):eaar5551.
- Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 2012; 22(1): 50-60.
- Subei AM, Cohen JA: Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs 2015; 29(7): 565-575.
- Chaudhry BZ, Cohen JA, Conway DS: Sphingosine 1-phosphate receptor modulators for the treatment of multiple sclerosis. Neurother J Am Soc Exp Neurother 2017; 14(4): 859-873.
- Pérez-Jeldres T, Alvarez-Lobos M, Rivera-Nieves J: Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs. 2021; 81(9):985-1002.
- Zecca C, et al: Half-dose fingolimod for treating relapsing-remitting multiple sclerosis: observational study. Mult Scler 201; 24: 167-174.
- Yamout BI, Zeineddine MM, Sawaya RA, Khoury SJ: Safety and efficacy of reduced fingolimod dosage treatment. J Neuroimmunol 2015; 285: 13-15.
- Kappos L, et al: A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387-401.
- Calabresi PA, et al: Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545-556.
- Cohen JA, et al: Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402-415.
- Langdon DW et al: Baseline characteristics and effects of fingolimod on cognitive performance in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 2021; 28(12):4135-4145.
- Fischer-Barnicol B, et al: Combination of teriflunomide and interferon as follow-up therapy after fingolimod-associated PML. Neurol Neuroimmunol Neuroinflamm 2021; 8. doi: 10.1212/NXI.0000000000000927
- Kappos L, et al: Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018; 391: 1263-1273.
- Selmaj K, et al: Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol 2013; 12: 756-767.
- Gajofatto A: Spotlight on siponimod and its potential in the treatment of secondary progressive multiple sclerosis: the evidence to date. Drug Des Devel Ther 2017; 11: 3153-3157.
- Huth F, Gardin A, Umehara K, He H: Prediction of the Impact of Cytochrome P450 2C9 Genotypes on the Drug-Drug Interaction Potential of Siponimod With Physiologically-Based Pharmacokinetic Modeling: A Comprehensive Approach for Drug Label Recommendations. Clin Pharmacol Ther 2019; 106:1113-1124.
- Gold, et al: Siponimod vs placebo in active secondary progressive multiple sclerosis: a post hoc analysis from the phase 3 EXPAND study.J Neurol 2022; 269(9): 5093-5104.
- Lamb YN: Ozanimod: First Approval. Drugs 2020; 80:841-848.
- Comi G, et al: Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol 2019; 18: 1009-1020.
- De Luca J et al: Effect of Ozanimod on Symbol Digit Modalities Test Performance in Relapsing MS. Mult Scler Relat Disord. 2021; 48:102673.
- D’Ambrosio D, Freedman MS, Prinz J: Ponesimod, a selective S1P1 receptor modulator: a potential treatment for multiple sclerosis and other immune-mediated diseases. Ther Adv Chronic Dis 2016; 7: 18-33.
- Olsson T, et al: Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. J Neurol Neurosurg Psychiatry 2014; 85: 1198-1208.
- Kappos L, Fox RJ, Burcklen M, et al: Ponesimod Compared With Teriflunomide in Patients With Relapsing Multiple Sclerosis in the Active-Comparator Phase 3 OPTIMUM Study: A Randomized Clinical Trial. JAMA Neurol 2021; 78(5): 558-567.
- Freedman MS, Pozzilli C, Havrdova EK, et al: Long-term Treatment With Ponesimod in Relapsing-Remitting Multiple Sclerosis: Results from Randomized Phase 2b Core and Extension Studies. Neurology 2022 Jun 6;10.1212/WNL.00000000200606.
- Ruggieri S, Quartuccio ME, Prosperini L: Ponesimod in the Treatment of Relapsing Forms of Multiple Sclerosis: An Update on the Emerging Clinical Data. Degener Neurol Neuromuscul Dis 2022; 12: 61-73.
InFo NEUROLOGY & PSYCHIATRY 2022; 20(5): 12-18.