Radiation-associated angiosarcoma is more common than intuitively expected. The initial diagnosis should be made as early as possible. Paradoxically, even in this radiation-induced tumor, re-irradiation is a good therapeutic option.
Radiation-associated angiosarcoma (RAAS) is so rare that there are no prospective studies. Therefore, all previous findings are based on retrospective case series and case reports. Because of the selective publication of the cases, treatment recommendations based on them should be made with caution. On the other hand, one must be content with this until larger case numbers and long-term observations from tumor registries, some of which are only being established, are available.
Angiosarcomas are highly malignant tumors of endothelial cells that account for only about 1-2% of all soft tissue sarcomas [1]. Angiosarcoma of the breast may be primary or secondary to chronic lymphedema after mastectomy (Stewart-Treves syndrome) [2] or in the setting of RAAS within a previous radiation field [3]. Primary angiosarcomas appear to have a better prognosis than RAAS [4–6]. Cahan’s modified criteria are usually used to distinguish them [7,8]. These include:
- Different histology of the initial primary tumor and the secondary tumor
- Emergence of the secondary tumor in the previous irradiation field
- Latency between the primary and secondary tumors of more than three years.
Generally, a median latency of six years between radiotherapy (RT) of the primary tumor and the onset of RAAS is reported [1,9]. This time span is much shorter compared to other radiation-associated sarcomas, which usually take 10-20 years to appear [10,11]. Some genetic disposition can be suspected, although not proven [12].
An analysis of the SEER database of nearly 275,000 breast cancer patients showed a cumulative incidence of RAAS of 0.09% at 15 years [9]. This appears to be increasing due to adjuvant RT routinely performed after breast-conserving therapy (BET) for breast carcinoma or DCIS [1]. The extent to which intensified systemic therapies contribute to the genesis remains unclear.
The 5-year survival rate is low and reported to be 27-35% based on the SEER database [9]. There are many reasons for this poor prognosis. The reddish-livid, partly two-dimensional discolorations appearing in the former irradiation field attract the patient’s attention too late. In this case, in addition to macroscopic infestation, occult “flea jump” microscopic metastasis in the skin occurs early on. This is difficult to counteract surgically, as it can quickly manifest again after excision of the macroscopic finding at a different site [10,13,14].
Uniform therapy recommendations do not exist. The focus is on the surgical procedure. The largest review to date based on 222 patients with a RAAS shows a 5-year local recurrence-free interval of 32% and a 5-year survival rate of 43% [1], which is higher compared with the SEER database (27-35%). This is likely because the patients analyzed were from case reports and case series with predominantly exceptional response and combination therapies, and thus the prognosis is overestimated due to publication bias. Conversely, this may indicate a possible improvement in prognosis with combination therapies.
Case Report
We report on a now 79-year-old female patient with a RAAS. 11/2006 was diagnosed with invasive ductal carcinoma of the breast on the left side. BET was performed with tumorectomy and axillary sentinel lymphadenectomy with a stage of pT1c pN0 (0/1 sn) cMO ER/PR positive, HER2 negative. Postoperative adjuvant normofractionated RT of the entire breast was performed with tangential fields up to 50 Gy and a local boost of 16 Gy followed by hormone therapy.
08/2011 the patient noticed a large livid-yellowish discoloration in the entire left breast with flare-ups of lumps and incipient exulceration. The nipple was already no longer recognizable (Fig. 1). Histologically, a poorly differentiated (G3) hemangiosarcoma was detected and confirmed by a second evaluation.
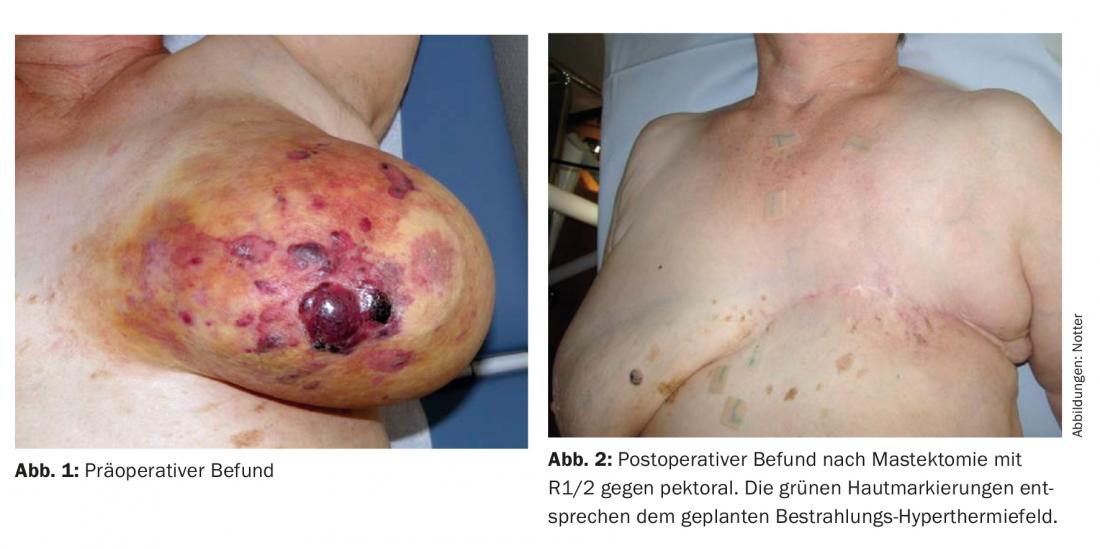
Preoperative discussion took place at the tumor board regarding therapeutic strategy, and mastectomy followed by re-irradiation (re-RT) combined with large-scale hyperthermia (HT) was recommended. Postoperatively, R1/R2 resection was seen in the area of the scar and against pectoral (Fig. 2) . A postresection was not performed. Subsequently, hypofractionated re-RT with 5× 4 Gy, 1×/week, concomitant with surface HT was performed in analogy to an established therapeutic regimen of breast and chest wall recurrences [15] ( Fig. 3) .
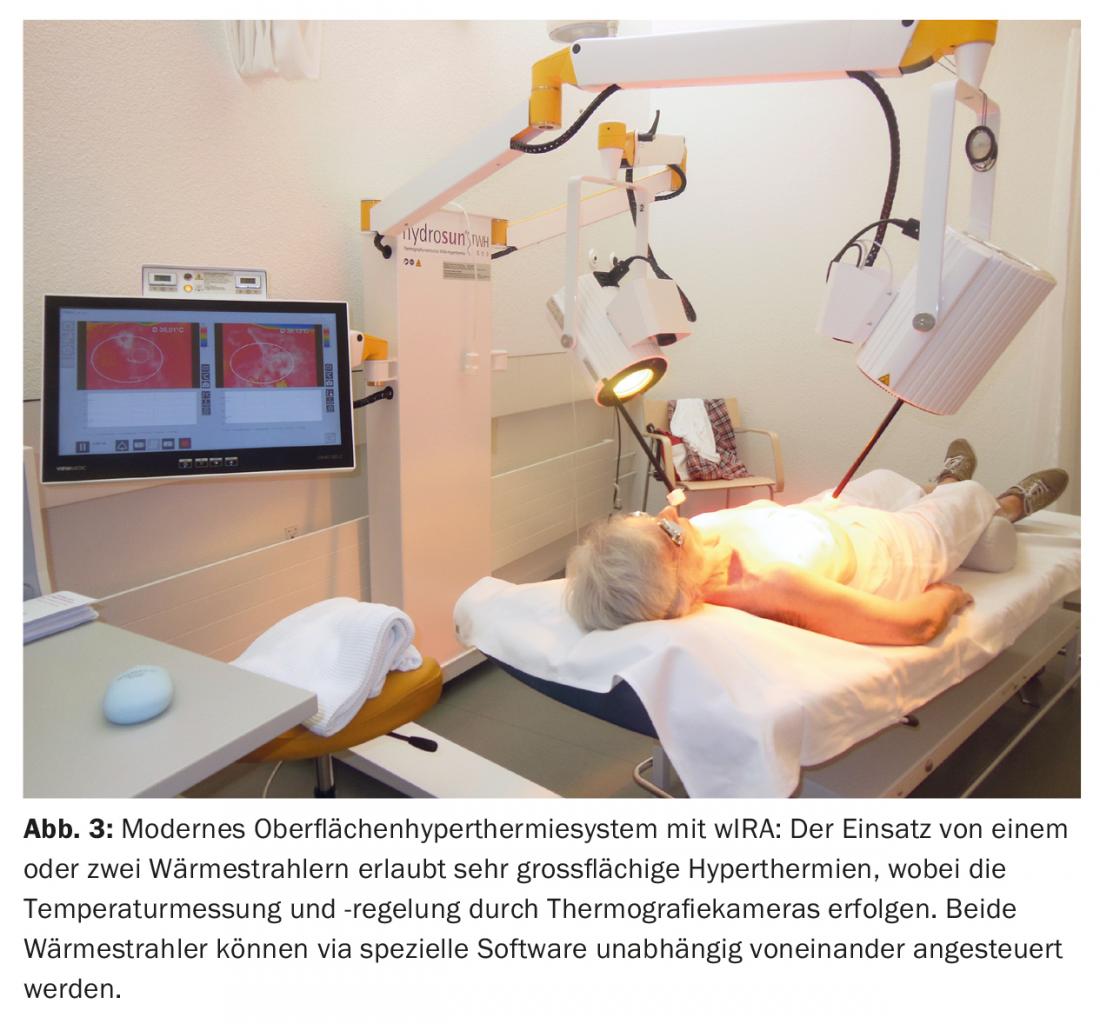
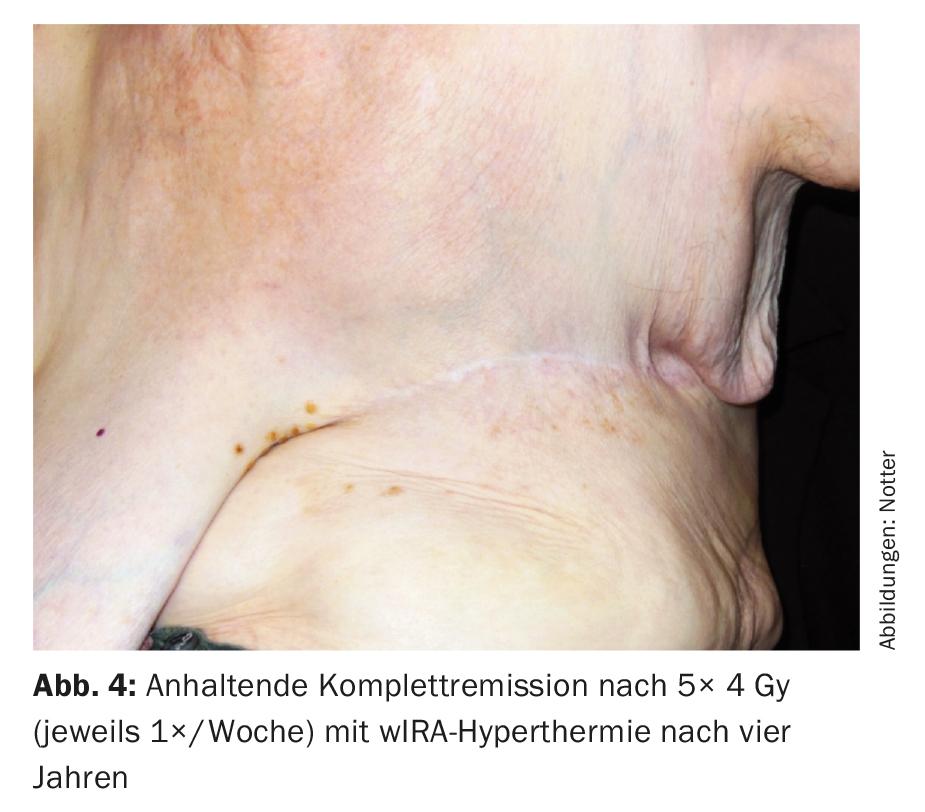
This was always administered on the same day before RT with thermography-controlled water-filtered infrared A (wIRA) for 45 minutes. RT was performed within two minutes after completion of HT with 4 Gy (9 MeV electrons) each and a total dose of 20 Gy. The primary target was the R1/R2 region, with large-scale irradiation of the entire left chest wall in prophylactic intention to treat occult metastases. This treatment was well tolerated, with only transient mild skin redness (CTCAE grade 1) as a side effect. Complete remission was also seen four years later (Fig. 4) and continued until six years later (08/2017). This is exceptional in that relevant parameters (R1/2 resection, tumor >10 cm, multifocality, G3) would suggest a very high local recurrence risk.
Discussion
Due to its rarity, RAAS of the breast is usually diagnosed too late and often not pre-discussed in an interdisciplinary manner initially/preoperatively. The standard is surgical R0 resection as part of a mastectomy or local chest wall resection with generous safety margins, although the risk of local recurrence is substantial at up to 66% despite R0 radicality [13]. Various approaches to reduce the risk of local recurrence have been described with even more extensive resections (“superradical”/”wide-margins”) and corresponding functional deficits and mutilations. It has been shown that R0 resection is prognostic both at initial surgery and in case of recurrence, but recurrences can arise from microscopically disseminated cells despite R0 resection [13,14,16]. The extent to which resection distance is a contributing factor in healthy individuals remains unclear. There are reports that could not demonstrate a prognostic difference between a free resection margin <1 cm and >1 cm [14]. A recent retrospective surgical study of 76 patients showed that “radical” surgery, with at least all previously irradiated skin removed, had a significantly better prognosis than “conservative” surgery (5-year local recurrence rate of 23% vs. 76%) [16]. However, more wound complications occurred. It should also be mentioned that significantly more chemotherapy (ChT) was also given concomitantly in the “radical” surgeries, which may have acted as a confounder.
Ultimately, control of occult microscopic metastases in the “area at risk” appears to be the primary prognostic determinant as a basis for subsequent recurrence. Thus, the question arises whether the control of the large-area “area at risk” must necessarily be performed surgically and whether there might not be less mutilating options. Options would therefore include both neoadjuvant and adjuvant chemotherapy (ChT). In the largest analysis involving RAAS and ChT with 95 patients, surgery alone was retrospectively compared with additional (neo)adjuvant ChT. Re-RT was considered contraindicated. With preoperative small chance of R0/R1 resection, neoadjuvant ChT was performed. Postoperative adjuvant ChT was performed for large tumor lesions, G3, or resection distances <1 cm. Surprisingly, the prognostically worse group with surgery and ChT showed a significantly improved 5-year local recurrence-free survival of 62.8% compared with 36.9% with surgery alone, but without a significant impact on 5-year survival rates [10]. Based on these data, unfortunately, no safe recommendation for ChT can be given, because on the one hand no uniform ChT regimen was used and on the other hand a subgroup analysis is not possible due to the too small number of cases. An additional limiting factor to be considered is that very many female patentees would hardly tolerate aggressive ChT due to their age and general condition.
May re-RT be offered despite a significant pre-existing condition? For various clinics and tumor boards, preload is still mistakenly considered a contraindication due to potential toxicities [10,14,16]. As shown by the example of re-RT (with HT) of breast and chest wall recurrences, this is not justified [15,17–20]. The side effect profile depends on fractionation, total dose, and latency between primary RT and planned re-RT. Data to date appear to support the use of re-RT in RAAS [21]. Of interest, analogous to classic chest wall recurrences, is the combination of re-RT with surface hyperthermia (HT).
In recurrent breast carcinoma, a meta-analysis of 2110 patients demonstrated a complete remission rate of 60% with a low adverse event rate when combining re-RT and HT [19]. Hyperthermo/re-radiotherapy (HTreRT) for breast or chest wall recurrence is included in the NCCN guidelines as “category 3 evidence.” HT is defined as a controlled heating of the tissue to a temperature of 39-43°C and acts primarily as a radio-sensitizer through
- Inhibition of repair of radiation-induced DNA damage
- Enhancement of tumor microperfusion with sensitization of hypoxic and thus radioresistant cells [19].
The latter is particularly useful in a radiogenically preloaded fibrosed and thus capillary poorly supplied chest wall [15].
In a review of 222 patients with RAAS, surgery with adjuvant re-RT achieved better local control than surgery alone. However, HT was given in addition to re-RT in 30% of cases [1].
In RAAS, a good response to the aforementioned combined HTreRT has been described, using different RT regimens and a different HT application than ours [22,23]. Patients after multiple recurrences were included, resulting in a low 5-year survival rate of 11% at a median age of 70 years, but a good palliative effect with 3-year control rates of 46% (postoperative) and 22% (HTreRT alone).
Conclusion
In summary, a direct comparison of different therapeutic options is not reliably feasible due to the low case numbers and retrospective data collection. In an entity such as RAAS of the breast, where one often “chases” the disease surgically, proactive well-coordinated interdisciplinary therapy should be sought. It would be advantageous if postoperative HTreRT were discussed at the time of diagnosis to cover the “area at risk” early. RAAS belongs on a specialist tumor board.
Take-Home Messages
- Radiation-associated angiosarcoma (RAAS), with an incidence of approximately 0.1%, is more common in irradiated breast cancer patients than intuitive
- expected and occurs much earlier than other radiation-associated sarcomas.
- The initial diagnosis should be made as early as possible and the combination therapy should be initially planned in an interdisciplinary manner at an experienced tumor board.
- Paradoxically, even in this radiation-induced tumor, re-irradiation is a good therapeutic option with a low side effect profile.
- There is much to be said for interdisciplinary therapy with surgery and adjuvant semi-prophylactic large-field hyperthermo-radiotherapy for RAAS of the chest/chest wall.
- The value of additional neo- or adjuvant systemic therapy requires further evaluation.
Literature:
- Depla AL, et al: Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: a systematic review. Eur J Cancer 2014; 50: 1779-1788.
- Stewart FW, Treves N: Lymphangiosarcoma in postmastectomy lymphedema; a report of six cases in elephantiasis surgica. Cancer 1948; 1: 64-81.
- Huang J, Mackillop WJ: Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer 2001; 92: 172-180.
- Fraga-Guedes C, et al: Primary and secondary angiosarcomas of the breast: a single institution experience. Breast Cancer Res Treat 2012; 132: 1081-1088.
- Fury MG, et al: A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005; 11: 241-247.
- Vorburger SA, et al: Angiosarcoma of the breast. Cancer 2005; 104: 2682-2688.
- Cahan WG, et al: Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1948; 1: 3-29.
- Arlen M, et al: Radiation-induced sarcoma of bone. Cancer 1971; 28: 1087-1099.
- Yap J, et al: Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys 2002; 52: 1231-1237.
- Torres KE, et al: Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol 2013; 20: 1267-1274.
- Manner J, et al: MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 2010; 176: 34-39.
- Nestle-Kramling C, et al: [Hemangiosarcoma after breast-conserving therapy of breast cancer: report of four cases with molecular genetic diagnosis and literature review]. Strahlenther Onkol 2011; 187: 656-664.
- Seinen JM, et al: Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol 2012; 19: 2700-2706.
- Lehnhardt M, et al: [Radiation-induced angiosarcoma of the breast]. Handchir Microchir Plast Chir 2017; 49: 103-110.
- Notter M, Piazena H, Vaupel P: Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: a retrospective study of 73 patients. Int J Hyperthermia 2016; 1-10.
- Li GZ, et al: Cutaneous Radiation-associated Breast Angiosarcoma: Radicality of Surgery Impacts Survival. Ann Surg 2017; 265: 814-820.
- Wahl AO, et al: Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys 2008; 70: 477-484.
- Vernon CC, et al: Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731-744.
- Datta NR, et al: Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2016; 94: 1073-1087.
- Datta NR, et al: Hyperthermia and reirradiation for locoregional recurrences in preirradiated breast cancers: a single institutional experience. Swiss Med Wkly 2015; 145: w14133.
- Ghareeb ER, et al: Primary and Radiation-induced Breast Angiosarcoma: Clinicopathologic Predictors of Outcomes and the Impact of Adjuvant Radiation Therapy. Am J Clin Oncol 2016; 39: 463-467.
- Linthorst M, et al: Effect of a combined surgery, re-irradiation and hyperthermia therapy on local control rate in radio-induced angiosarcoma of the chest wall. Strahlenther Onkol 2013; 189: 387-393.
- de Jong MA, et al: Reirradiation and hyperthermia for radiation-associated sarcoma. Cancer 2012; 118: 180-187.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(4): 28-32.











