Dosages in pediatrics pose a great challenge to pediatric physicians because in many cases no binding data are available. This is also the case in phytotherapy, because binding data can only be found with corresponding clinical studies, which are hardly ever carried out for ethical reasons, or permits are hardly ever granted for them.
Phytotherapy suitable for children
Phytotherapy offers itself as a suitable form of therapy for children for several reasons. Many herbal medicines have a wide therapeutic range, so the risk of overdose in the majority of cases is very small. Because of their usually mild effects, many medicinal plants are suitable for pediatric applications, either as a single treatment or as an additional treatment. In addition, herbal medicines are very suitable for indications that often need to be treated in children, such as respiratory tract diseases, gastrointestinal tract complaints, restlessness and sleep disorders, as well as dermatological applications. It is of great advantage if professionals such as physicians and pharmacists have sufficient information for the dosage of herbal medicinal products, so that they can optimally advise parents and safely use herbal medicinal products in appropriate indications.
Dosage basics
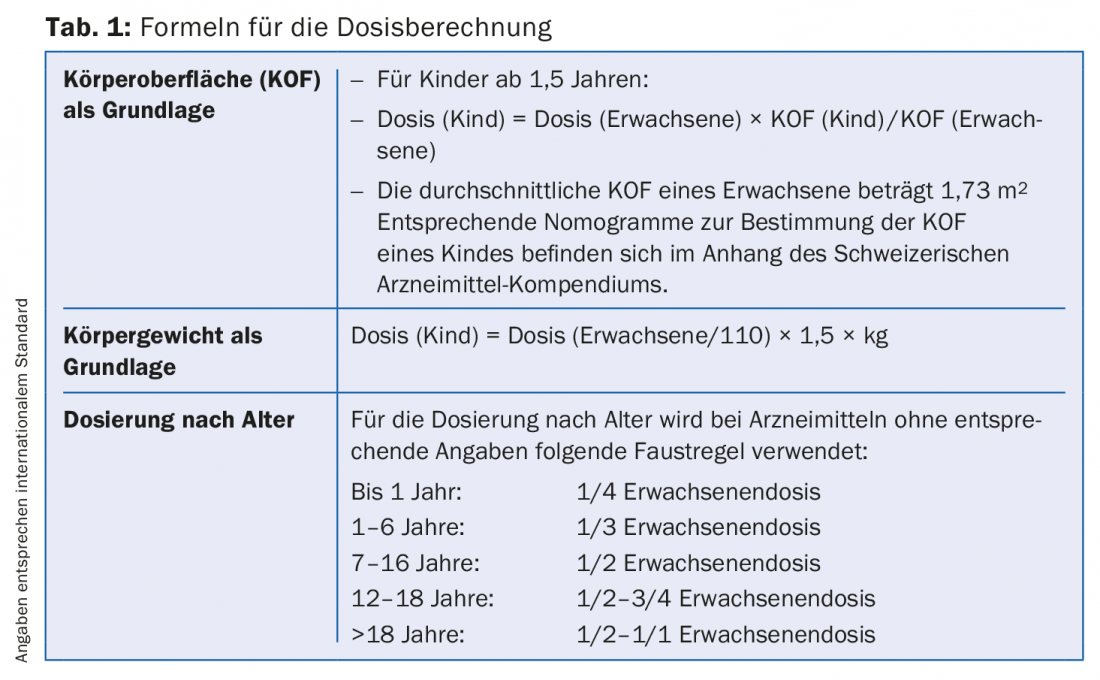
To calculate children’s dosages of medicines, formulas are used that try to match the nature of the child (Tab. 1). There is little literature on the dosage of herbal medicines in children [1–3]. If information about children’s dosages is listed in the package insert of a drug, this information naturally applies, because it is approved by Swissmedic. Table 2 lists the herbal medicinal products on the Specialty List (SL) for which children’s dosages are indicated in the package leaflet.
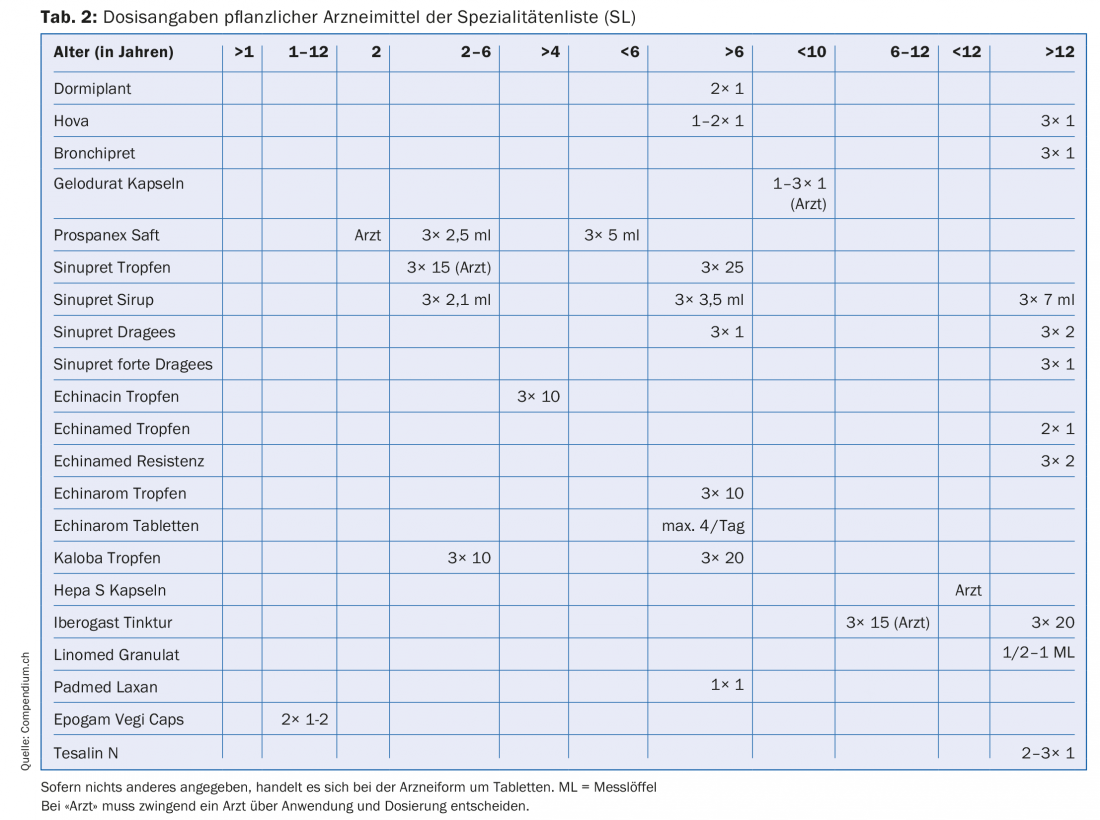
The ages
Table 2 shows that there are dosage indications for quite different age groups. Age groupings range from >1 year to >12 years with numerous sub-groupings in between.
In addition, there is patient information that mandates the involvement of a physician regarding a planned use for certain age groups. Ex. Prospanex cough syrup: in infants under two years of age, only a doctor should decide on the use and dose. There are also medicinal products for which the patient information provides dose information for a specific age group, but still refers to the physician, who is ultimately responsible. This is the case, for example, with Iberogast drops for children 6-12 years.
Why are there such different age groups? There are several reasons for this.
European Medical Agency (EMA)
The European Medical Agency, the London-based registration authority of the European Union, stipulates that when a drug is newly approved, the drug in question is contraindicated in children and adolescents under 12 years of age, unless the applicant can provide appropriate clinical data [4].
So if a pharmaceutical company wants to register uses in children as part of an approval or renewal of an approval for a drug, it must submit what is known as a Paediatric Investigation Plan (PIP). This document, created by the EMA, regulates exactly what documentation and what type of studies are required for such approval. A submitted PIP is reviewed by the Paediatric Committee (PDCO), an organization of the EMA. The PDCO decides on approvals for these children’s applications.
International Conference on Harmonisation (ICH)
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, an organization founded in 1990, is responsible for defining the age classes of children. According to its website, its purpose is to achieve better harmonization in the development and registration of effective and safe medicines worldwide. It promotes dialogue between manufacturers and registration authorities. In the Guidelines “Clinical Investigation of Medicinal Products in the Pediatric Population” [5] approved on July 20, 2000, ICH defines the following pediatric age groups:
- Newborn: 0-27 days
- Infants/Toddlers: 28 days to 23 months
- Children: 2-11 years
- Teenagers: 12 to 16-18 years
This classification is justified by differences in central nervous system (CNS) development, immune system, renal and hepatic clearance, and hormonal changes (adolescents) in these age groups.
Approval without PIP
As mentioned earlier, if a pharmaceutical company wishes to obtain a dosage recommendation for one or more of these age groups for a particular indication as part of the approval process for a drug, it must submit a PIP with appropriate information. Unless it does so, or the information in the PIP is approved, the EMA says the use of this drug is contraindicated for children and adolescents under 18 years of age. This is the case, for example, for the herbal medicine Kardionin, a Crataegus preparation that has been registered since 2013. Since the supplier of Kardionin was unable or unwilling to submit documentation for use in children, which is quite possible given the indication of the drug, the package leaflet states use only for adults >18 years of age is indicated, as well as the information “The use and safety of Kardionin in children and adolescents have not been studied.”
Deviating data
In the list of herbal medicines below (Tab. 2), which includes information on children’s dosages, there are many indications that do not correspond to the ICH age classification, for example, dosages for the children over six years of age or between 2-6 years of age. These claims may be made because the corresponding drugs received approval from the EMA and ICH before the regulations became effective and were allowed to make the corresponding claims based on the regulations at that time.
Summary
Since herbal medicines are well suited for use in pediatrics, it is important that professionals have a reliable basis for dosing appropriate age groups. In the absence of such information, approximations of appropriate dosages can be made using the child’s body surface area, weight, or age. However, these are approximations and must be handled with care. In the case of a new registration of a drug, i.e. also of a herbal drug, the applicant must, according to the regulations of the EMA and the ICH, provide accurate information on the efficacy and safety of the drug, substantiated with clinical studies, so that corresponding claims may be made. Otherwise, the drug is contraindicated in children and adolescents <18 years.
Literature:
- Schicher H, Dorsch W: Phytotherapy in pediatrics. Wissenschaftliche Verlagsgesellschaft Stuttgart 2006.
- Kooperation Phytopharmaka, Bonn: “Kinderdosierungen von Phytopharmaka” – 3rd revised and expanded edition 2002.
- von Mandach U, et al: Application of herbal medicinal products in pediatrics. Phytotherapy 2002(5); 2: 8-16.
- www.ema.europa.eu
- www.ich.org
HAUSARZT PRAXIS 2017; 12(8): 2-4