The pathomechanisms of atopic dermatitis (AD) are becoming increasingly better understood. A genetically determined immunological imbalance is characterized by an increased Th2 response and is associated with the production of inflammatory cytokines such as interleukin (IL)-4 and IL-13. There are also other molecules that play an important role in the inflammatory process. The therapy landscape is changing. Two biologics and three Janus kinase inhibitors are currently approved in the indication area of AD in Switzerland and more are in development.
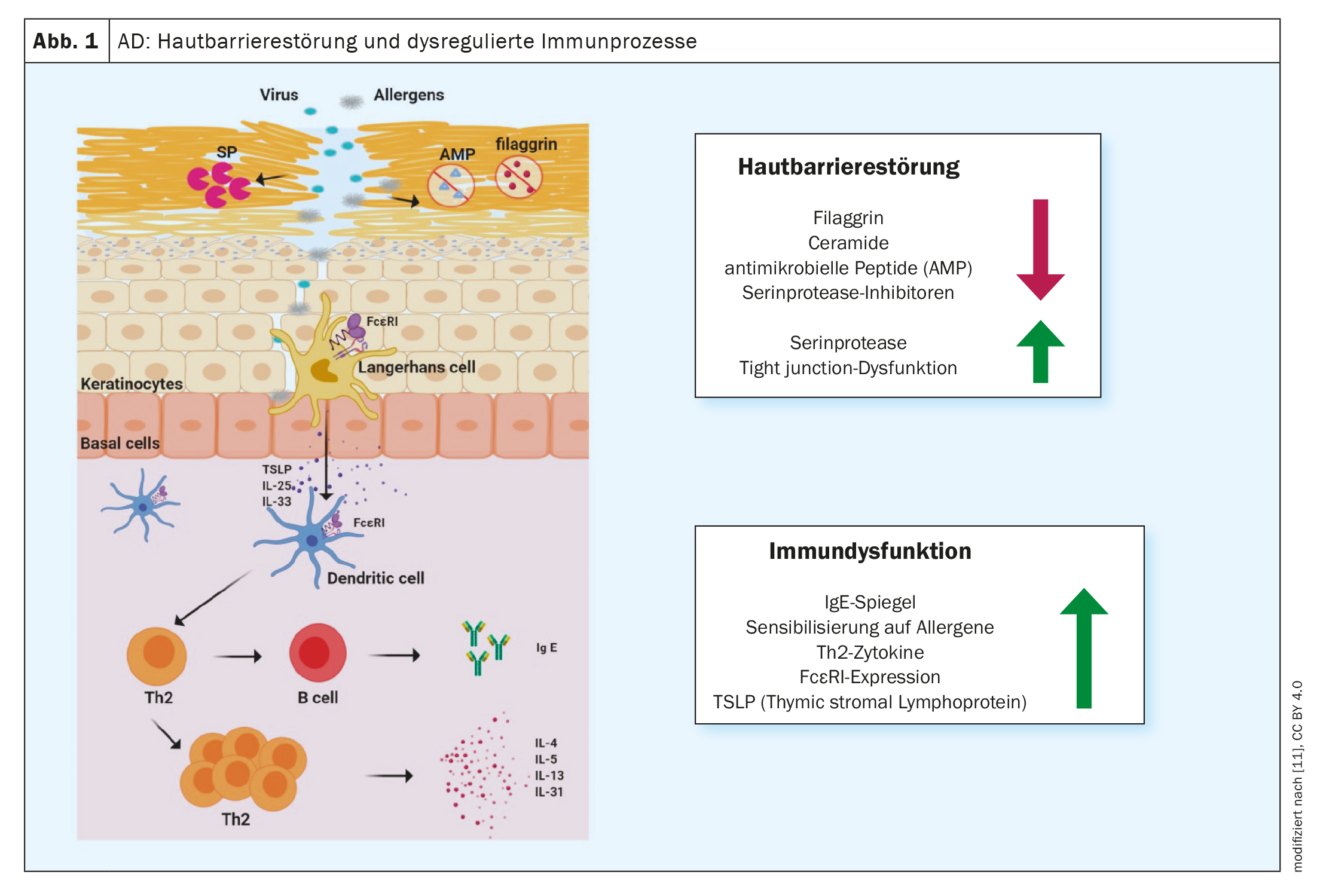
Type 2 inflammation is a common cause of various allergic diseases, including atopic dermatitis (AD), bronchial asthma, chronic rhinosinusitis, eosinophilic esophagitis and allergic rhinitis [1]. More and more effective treatment methods are being developed in the fight against allergic diseases. Monoclonal antibodies and Janus kinase (JAK) inhibitors have made a significant contribution to improving treatment options, emphasized Prof. Peter Schmid-Grendelmeier, MD, allergy expert, Department of Dermatology, University Hospital Zurich, at the annual congress of the Swiss Society of Allergology and Immunology [2]. This encouraging development was preceded by intensive research into the pathophysiological processes. It is now known that a dominant T helper (TH)2 immune response is associated with the secretion of cytokines such as interleukin (IL)-4, IL-5 and IL-13 (Fig. 1). This inflammatory milieu not only promotes the generation, maturation and activation of eosinophil and basophil granulocytes, but also the activation of mast cells [3].
Molecular basis of type 2 immune reactions
In some cases, allergic diseases also occur comorbidly – for example, there are patients who have severe asthma or AD and also suffer from allergic rhinitis. Such comorbidities can further increase the burden of disease. Type 2 inflammation is maintained by TH2, innate lymphoid cells type 2 (ILC2) and mediators of the innate and adaptive immune system [1]. Empirical studies show that 58-79% of all adult AD patients suffer from at least one other atopic disease, regardless of severity [4–6].
Type 2 immune responses are the cellular correlate of skin inflammation in AD and also significantly influence barrier function and microbial dysbiosis [7]. “We now know a lot about atopic dermatitis,” summarized the speaker. A few years ago this was merely ‘nice to know’, but in the meantime the increasing understanding of the pathophysiological relationships has led to several innovative immunomodulatory therapy options. In contrast to biologics, Janus kinase (JAK) inhibitors do not intercept cytokine signals in the extracellular space, but intracellularly.
Biologics and JAK inhibitors on the rise
The first biologic approved in the AD indication was dupilumab (Dupixent®) in 2019. This is a monoclonal antibody directed against the α-subunit of the IL-4 receptor, which blocks the signals of the inflammatory mediators IL-4 and IL-13 [8,9]. This treatment approach has proven to be very effective, according to Prof. Schmid-Grendelmeier. Tralokinumab (Adtralza®), another biologic that specifically neutralizes IL-13, has been available for AD patients since 2022 [8]. Although there is currently less extensive real-world evidence available compared to dupilumab, the evidence of efficacy to date has been very convincing.
JAK inhibitors represent another promising therapeutic approach. In Switzerland, three JAK inhibitors – baricitinib (Olumiant®), abrocitinib (Cibinqo®) and upadacitinib (Rinvoq®) – have recently passed the approval hurdles in the AD indication [8]. JAK inhibitors exert their effect via the intracellular JAK-STAT mechanism.
| As antibodies or fusion proteins, biologics block a single cytokine extracellularly or a cytokine receptor or a surface molecule on the cell, whereas JAK-i act intracellularly and modulate various cytokines. In contrast to biologics, the aim of JAK-i is to reversibly reduce the activity of one or more JAK isoforms, similar to turning down a thermostat. The effect is therefore broader and less specific. |
| to [12] |
Criteria-based therapy decision
“A biologic targets one or two cytokines and blocks them more or less completely. A JAK-i, on the other hand, reduces several cytokines with different affinities. There are pros and cons for both approaches,” explained Prof. Schmid-Grendelmeier [2]. The selection of the respective therapy option should be individually tailored to the patient, taking into account aspects such as concomitant diseases, infection status and age. Situational factors can also be included in these considerations; for example, JAK-i tends to have a faster onset of action, but in contrast to biologics requires relatively extensive screening and laboratory monitoring in parallel with therapy [10]. Another important difference for patients’ everyday lives concerns the form of administration: baricitinib, abrocitinib and upadacitinib are taken orally as tablets once a day, whereas the biologics dupilumab and tralokinumab are administered as injections at intervals of a few weeks or months. The extended treatment spectrum for AD is now also reflected in the updated version of the S3 guideline. While dupilumab was already included in the previous version of the guideline, tralokinumab, abrocitinib, baricitinib and upadacitinib are now also covered [9].
Conclusion and outlook
For patients with a high degree of AD severity, the new treatment options represent a major advance, according to the speaker [2]. But there is still a lot of research to be done. In Switzerland, dupilumab is so far the only representative from the group of newer system therapeutics (biologics, JAK-i) that has received an indication extension for pediatric AD patients aged 6 years and older. Tralokinumab is approved in Switzerland from the age of 18 and in the EU from the age of 12. In the case of JAK-i, approval has so far been limited to adult patients. But this could change in the not too distant future, especially as the use of several biologics and JAK-i in pediatric AD patients is currently being investigated.
In addition, further active substances with new targets are currently being researched. Lebrikizumab, nemolizumab, tezelumab and Ox-40-Ak have so far proven to be promising drug candidates [2]. Lebrikizumab binds specifically to freely circulating IL-13 and nemolizumab blocks the α-subunit of the IL-31 receptor. Tezelumab is an antibody against TSLP (thymic stromal lymphoprotein) and Ox-40 is a costimulatory receptor on activated T cells.
For patients with moderate to severe AD, an expansion of treatment options offers better chances of recovery, provided access to therapies is available. Basic care with emollients and patient education remain important pillars of treatment for AD of any severity [2]. Patients receive a lot of valuable information and advice on disease management during atopic dermatitis training sessions.
Congress: Swiss Society for Allergology and Immunology (SSAI), Annual Congress
Literature:
- Klimek L, et al: Type 2 inflammation: the value of different biologics in practice. Dtsch Arztebl 2021; 118(50): [20]; DOI: 10.3238/PersPneumo.2021.12.17.05
- “Novel concepts in Allergy”, Prof. Dr. med. Peter Schmid-Grendelmeier, SSAI Annual Congress, 24-25.08.2023.
- Kühn M, et al: TH2 immune response: significance and therapeutic influence. Swiss Med Forum 2021; 21(0102): 13-17.
- Weidinger S, et al: Atopic dermatitis. Nat Rev Dis Primers 2018; 4(1): 2.
- Chiesa Fuxench ZC, Ong P: Poster presented at AAD 2018. Poster 6236.
- Drucker AM, et al: The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J Invest Dermatol 2017; 137(1): 26-30.
- Lauffer F, Biedermann T: Atopic eczema: A disturbing fire for a triangular relationship. Dtsch Arztebl 2021; 118(24): [24]; DOI: 10.3238/PersDerma.2021.06.18.04
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch,(last accessed 15.11.2023)
- S3 guideline “Atopic dermatitis”, AWMF register no.: 013-027, status 17/06/2023.
- Worm M, et al: Modern therapy of atopic dermatitis: biologics and small molecule drugs. JDDG 2020; 18(10): 1085-1093.
- Yang G, et al: Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. International Journal of Molecular Sciences 2020; 21(8): 2867.
www.mdpi.com/1422-0067/21/8/2867,(last accessed 11/15/2023) - Choy EH: Clinical significance of Janus kinase inhibitor selectivity. Rheumatology (Oxford) 2019; 58(6): 953-962.
DERMATOLOGIE PRAXIS 2023; 33(6): 22-23 (published on 12.12.23, ahead of print)