The monoclonal antibody dupilumab blocks the common receptor component for interleukin (IL)-4 and IL-13, the central drivers of type 2 inflammation. It has been approved in Switzerland since 2019, and new studies have looked at its efficacy and safety in patients with moderate to severe COPD with T2 inflammation and in children with uncontrolled moderate to severe asthma. Results were presented at the DGP Congress 2024.
COPD exacerbations are associated with an increased risk of further exacerbations, a deterioration in lung function and high morbidity and mortality. COPD diseases with type 2 inflammation are particularly affected.
BOREAS was a 52-week, randomized-controlled, double-blind, phase 3 study of the efficacy and safety of biweekly dupilumab 300mg in COPD patients with blood eosinophils ≥300 cells/µl. Ideally, they should already have received triple therapy consisting of an inhaled corticosteroid (ICS), a long-acting β2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA). This applied to 98% of the population, as PD Dr. Henrik Watz, Pneumology Research Institute at the Grosshansdorf Lung Clinic (Germany), explained [1]. In exceptional cases, such as when ICS was contraindicated, the combination LAMA/LABA was also permitted. The exacerbations should have already occurred during treatment as an indicator of the need for further therapy. A history of asthma was a criterion for exclusion.
939 patients with blood eosinophils ≥300 cells/µl were included in BOREAS and randomized in a 1:1 ratio to dupilumab 300 mg (n=468) or placebo (n=471) subcutaneously (SC) once every 2 weeks (q2w) for 52 weeks. The patients were between 40 and ≤80 years old. The primary endpoint was the annualized rate of moderate to severe exacerbations from baseline to week 52. Secondary endpoints included the change in FEV1value before bronchodilation at week 12 and week 52 compared to baseline (BL), cumulative exacerbations over time and the safety or health-related quality of life of patients on therapy as Patient Reported Outcome (PRO).
COPD exacerbation rate reduced by 30%
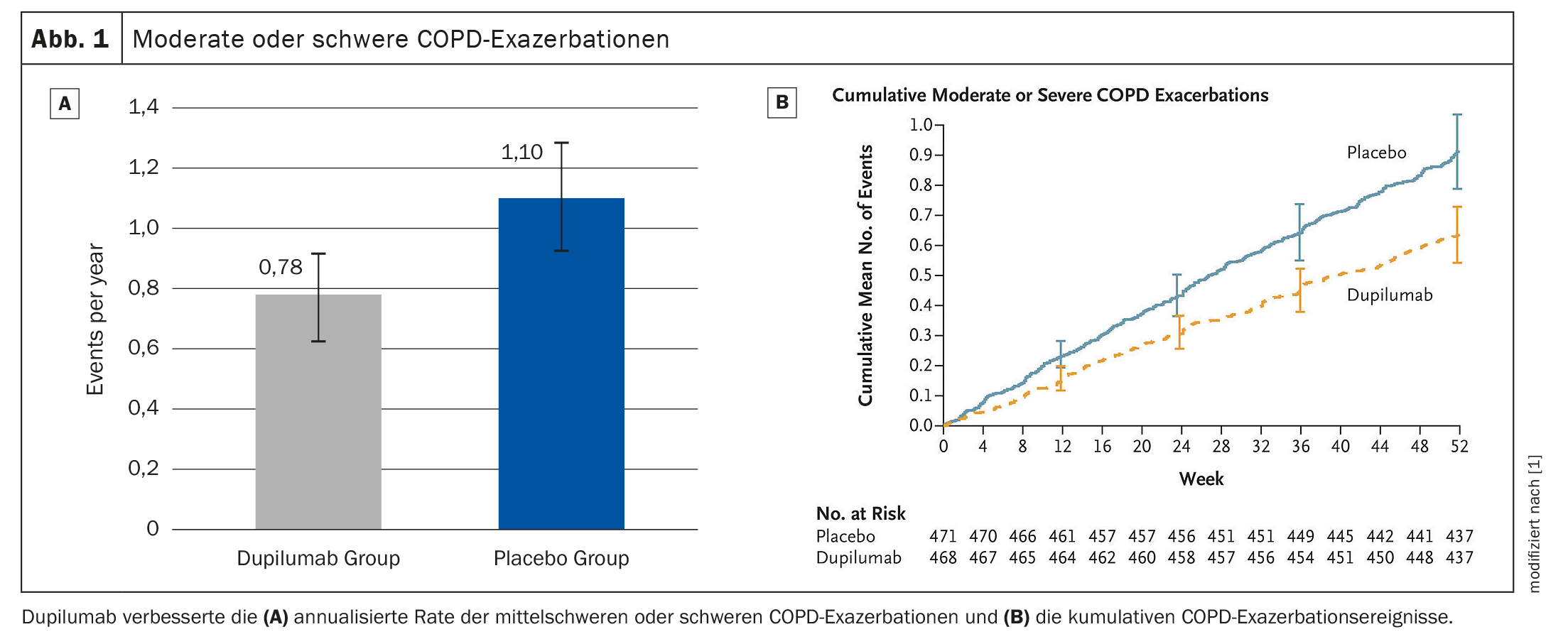
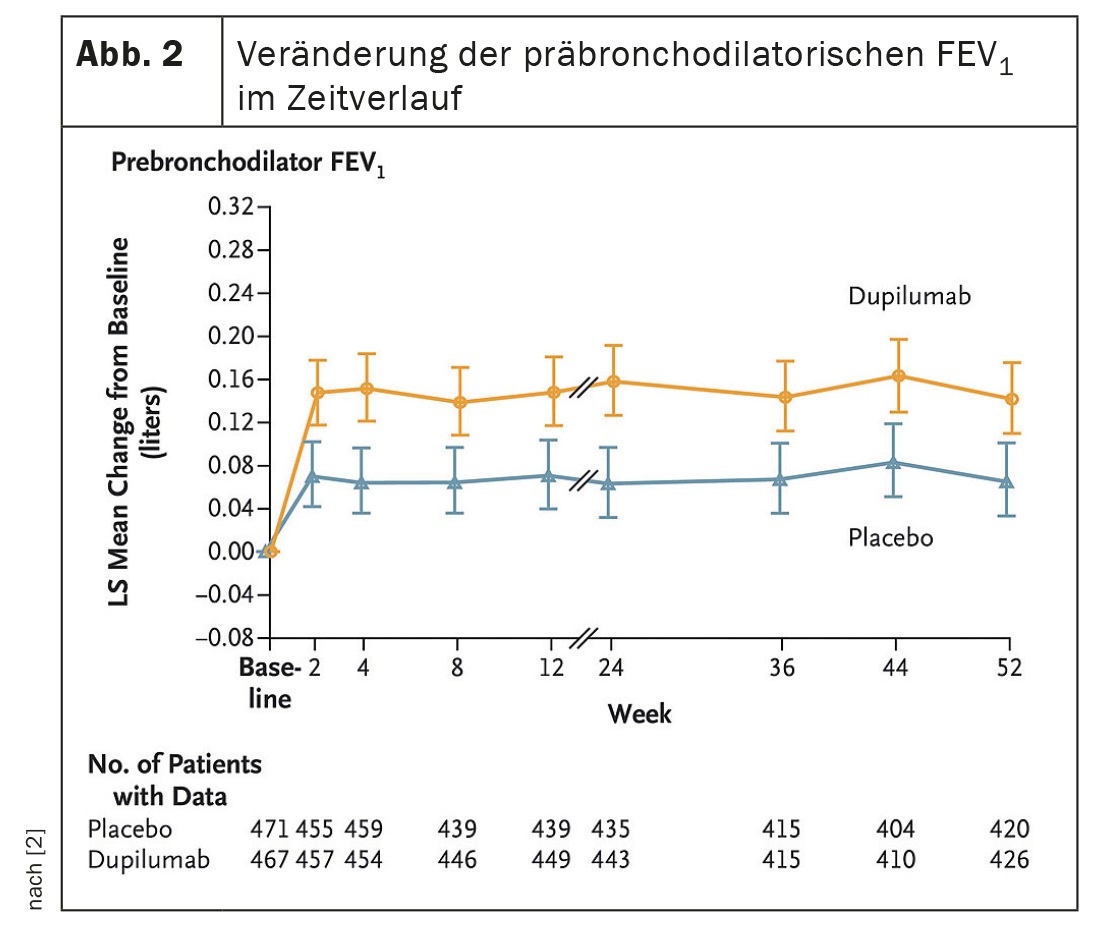
After 52 weeks, dupilumab reduced the exacerbation rate by 30% compared to the placebo group (p=0.0005) (Fig. 1A, 1B). The IgG4 antibody increased the pre-BD FEV1 value at week 12 was significant (LSM difference vs. placebo: 83 ml, p<0.0001); which persisted until week 52 (83ml, p=0.0003) (Fig. 2) . This tended to result in less annual exacerbation-associated treatment with systemic corticosteroids (SCS) in the dupilumab- (13.57 days [SD 13,17] compared to the placebo arm (19.09 days [SD 20,65]). Safety was similar in both groups, adverse events were balanced.
Dupilumab thus significantly improved moderate to severe exacerbations, lung function, quality of life and symptoms in COPD patients with T2 inflammation, Dr. Watz concluded. The use of SCS days required for the treatment of AECOPD tended to decrease with dupilumab.
Long-term effect on lung function in pediatric asthma patients
Minimizing the impairment of lung function is a primary goal in the treatment of paediatric asthma. Long-term use of dupilumab in the Excursion study resulted in a sustained improvement in lung function in 6-11 year old patients with uncontrolled moderate to severe type 2 asthma who completed the Voyage study; a rapid improvement in lung function was observed in patients who were switched from placebo (PBO) to dupilumab (DPL).
In Voyage, dupilumab significantly reduced severe exacerbations and improved lung function over 52 weeks. Excursion was an open, single-arm extension study with children who had previously participated in Voyage. Dr. Theresa Guilbert, pediatric pulmonologist at Cincinnati Children’s Hospital and University of Cincinnati (USA), presented a post-hoc analysis of Excursion that evaluated the numerically higher lung function improvements observed in the DPL/DPL vs. the PBO/DPL arms in Excursion [3].
The children received dupilumab 100 mg or 200 mg every 2 weeks according to body weight over a period of one year. The researchers looked at the change from baseline in the parent study (PSBL) and percent FEV1 before bronchodilation over 104 weeks, as well as the impact of baseline characteristics such as age at onset of asthma, time since diagnosis, inhaled corticosteroid dose (ISC) at baseline and asthma control on the efficacy of dupilumab.
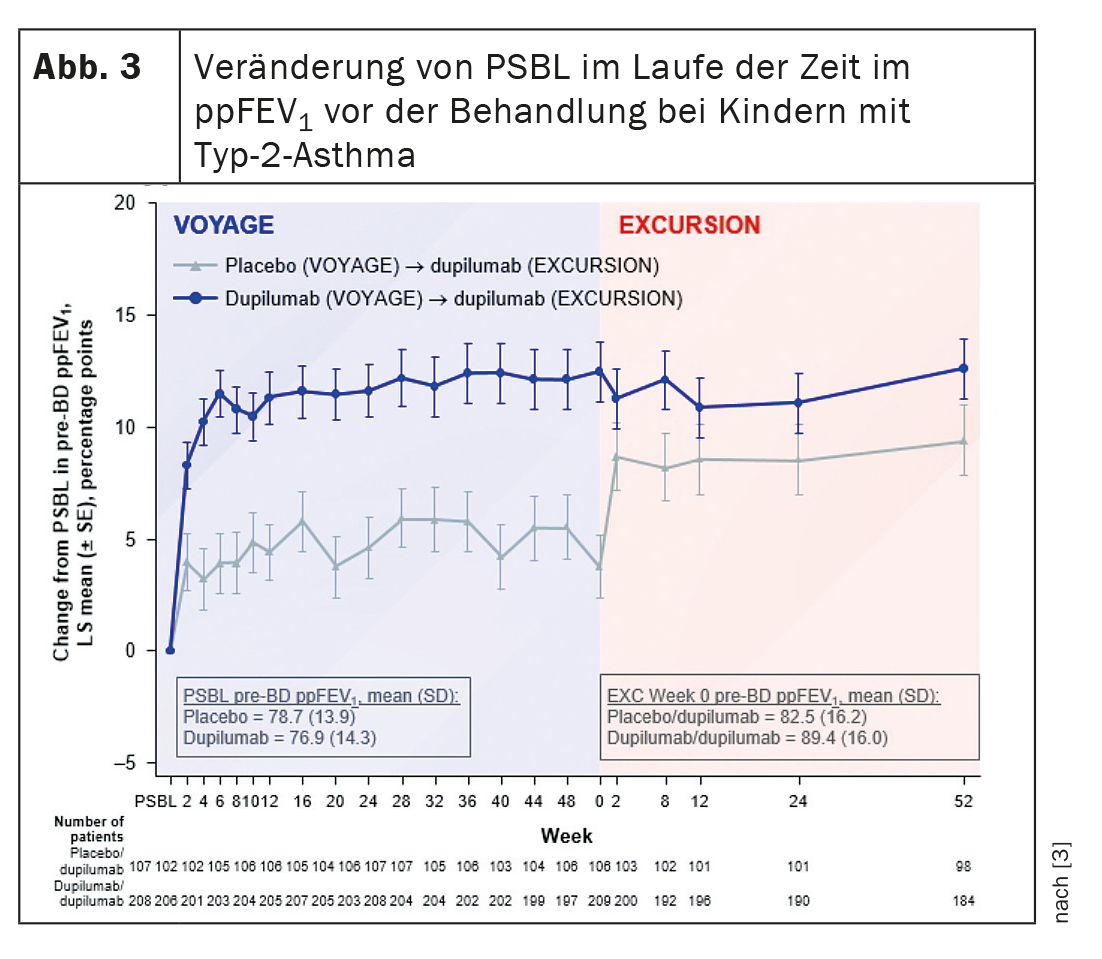
Dupilumab rapidly improved lung function during Voyage, which continued during Excursion. In Excursion, patients who received placebo during Voyage showed a rapid and sustained improvement in lung function from the start of dupilumab treatment (Fig. 3) . Dupilumab was able to improve and maintain the pre-bronchodilator predicted percent FEV1 up to 104 weeks, Dr. Guilbert summarized the results. The response of the lung function to the biologic over a period of 1 to 2 years did not differ according to the duration of the asthma or the age at the start of therapy.
The discussion with these data is that the patients who switched from placebo to dupilumab did not fully recover their lung function, noted Prof. Dr. Marek Lommatzsch, University Medicine Rostock (Germany). So the question is: Is this a real effect and does this mean that a one-year delay in therapy means a loss of lung function? Dr. Guilbert explained that her research group initially had the same idea. “However, this is not a statistical difference. The value is numerically higher, but it does not reach statistical significance compared to the placebo group.” Larger numbers are probably needed to determine this difference. In addition, “there is of course a lot of discussion about whether we should be talking about clinical remission in treatment with the advent of biologic therapies”. There is some debate as to whether or not this is appropriate in pediatrics.
Congress: DGP 2024
Sources:
- Watz H: Efficacy and Safety of Dupilumab for COPD with Type 2 Inflammation Indicated by Elevated Eosinophils. Session “News on clinical studies in asthma and COPD”. 64th Congress of the German Society for Pneumology and Respiratory Medicine e.V., 20-24.03.2024, Mannheim (D).
- Bhatt SP, Rabe KF, Hanania NA, et al: Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N Engl J Med 2023; 389: 205-214; doi: 10.1056/NEJMoa2303951.
- Guilbert T: Long-term Dupilumab Effect on Lung Function in Paediatric Patients with Uncontrolled Asthma. Session “News on clinical studies in asthma and COPD”. 64th Congress of the German Society for Pneumology and Respiratory Medicine e.V., 20-24.03.2024, Mannheim (D).
InFo PNEUMOLOGIE & ALLERGOLOGIE 2024; 6(2): 36-37 (published on 30.5.24, ahead of print)