Iron is essential for various biochemical and physiological functions. Iron deficiency is common and one of the main causes of anemia. As a prophylaxis, an adequate supply of ferritin through the diet is important. In the presence of iron deficiency, oral or intravenous substitution can be used, although efficacy and tolerability vary from individual to individual.
Iron is one of the four most common elements in the earth’s crust. It is an important trace element for all living things – including humans. It owes its essential role in biology to its ability to transport and reversibly bind electrons. In hemoglobin, for example, it is responsible for ensuring that the highly electronegative oxygen can be transported effectively. For the same reason, iron is a central component of numerous enzyme systems that perform many biochemical and physiological functions.
More than 1.2 billion people worldwide suffer from iron deficiency [1]. This is one of the main causes of anemia [2]. In Europe, data collections among women of childbearing age show an iron deficiency prevalence of 10-32%, with an estimated prevalence of anemia of 2-5%. The percentage of women with low iron stores is estimated to be 40-55% [3]. The prevalences for iron deficiency in young children were recorded locally and showed values of 4-48%. Thus, young children represent another risk group for iron deficiency [4].
Why is one of the most widespread elements at the same time responsible for a weighty global deficiency? The reason lies in the ability of iron to promote the formation of toxic reactive radicals. Furthermore, iron is virtually insoluble in free form at physiological pH (i.e., everywhere in the body except in gastric acid). The absorption of the iron is therefore naturally limited. For it to be utilized, it must be taken up in bound form: associated with proteins that can bind it strongly but reversibly.
Iron metabolism and absorption
To date, no active excretory mechanism for iron is known; renal excretion is irrelevant to iron balance. Human iron balance must therefore be actively regulated during absorption from food in the small intestine, where enterocytes play a key role.
Iron in food exists in two main forms: Haeme iron (in animal products) and non-haeme iron (in animal and non-animal products). It is primarily absorbed in the small intestine, where the pH of the intestinal contents successively increases along a gradient to near neutrality. Non-heme iron from food becomes more insoluble along this gradient, so absorption in the distal intestine probably plays a minor role. The solubility of iron in the small intestine is an important factor in the absorption of iron from food and can be significantly influenced by the composition of a meal.
At the cellular level, heme iron and non-heme iron are taken up by different transporters. Both contribute to the iron concentration in enterocytes, which is crucial for cellular iron regulation. It has a direct influence on the conformation of “Iron Response Proteins” (IRPs), which control the synthesis of cellular transport proteins. Thus, iron intervenes directly in the synthesis of the transport mechanisms that import or export it into the cell. This is particularly important for the control of systemic iron balance in enterocytes, macrophages and hepatocytes.
A second, systemically acting regulatory component is the hormone hepcidin, which is synthesized in the liver. Hepcidin is regulated by different signals: Increased erythropoiesis or hypoxia lead to low concentrations, whereas inflammatory/infectious stimuli (interleukin-6) and endogenous iron status (saturation of transferrin) increase synthesis. Hepcidin binds to the only known cellular iron exporter, ferroportin, leading to its degradation. Thus, the iron remains in the cells and can no longer enter the bloodstream, which in turn affects the IRPs and its reaction cascade. Since this process is systemically regulated, this affects all cell types. Thus, a balanced iron budget is the result of the processes of all individual cellular iron budgets and their systemic orchestration by hepcidin [5]. The discovery of this hormone in the 2000s has greatly expanded the understanding of iron regulation: chronic elevation of hepcidin results in marked anemia that cannot be corrected, or can only be partially corrected, by oral iron supplementation; for example, anemia caused by chronic inflammatory or infectious diseases.
Iron recovery through recycling
Iron in adults is ≈4 g and is found primarily in red blood cells (≈2.8 g). 90% of the daily requirement is covered by recovery from old red blood cells. This recycling cycle takes place between bone marrow, red blood cells and macrophages. In the bone marrow, the red blood cells (≈300 mg) are produced, and the macrophages take up the old erythrocytes, break them down, and release the iron back into the bloodstream, where it can again be used by the bone marrow (≈600 mg). Iron stores vary individually and are primarily found in the liver. The iron to be transported in the bloodstream is bound to transferrin, which is a dynamic but small fraction (3-4 mg) of the total iron content. This fraction is responsible for transport between cells and tissues and is therefore of great functional importance.
10-20% of the daily iron requirement (1-2 mg) must be taken in through food to compensate for natural daily iron losses (e.g. through minor bleeding, through mucous membranes, etc.). Since the amount to be absorbed per day is very small compared to the body content, iron deficiency can only result from a long-term iron deficit. Young women, pregnant women or children are most frequently affected, as blood loss during menstruation and growth lead to increased iron requirements.
Anemia increases the risk of pregnancy complications and can lead to preterm birth. Iron deficiency can be chronic and asymptomatic. Symptomatically, it manifests itself in fatigue, lack of concentration, irritability, decreased tolerance to cold, shortness of breath during physical activity, pica syndrome and restless legs syndrome. Other symptoms include skin and conjunctival pallor, stomatitis, and koilonychia.
Biomarker for iron deficiency
Biochemical measures of iron status reflect the relative size of the different compartments and at the same time inform about the overall iron status of the organism. The gold standard is the measurement of the iron content in the bone marrow. If this is too low, too little iron is recycled in the body’s circulation. this deficit cannot be compensated by diet or liver stores, resulting in reduced erythropoiesis and anemia.
An indication of iron deficiency anemia is also the increase in hemoglobin after iron administration in anemic patients.
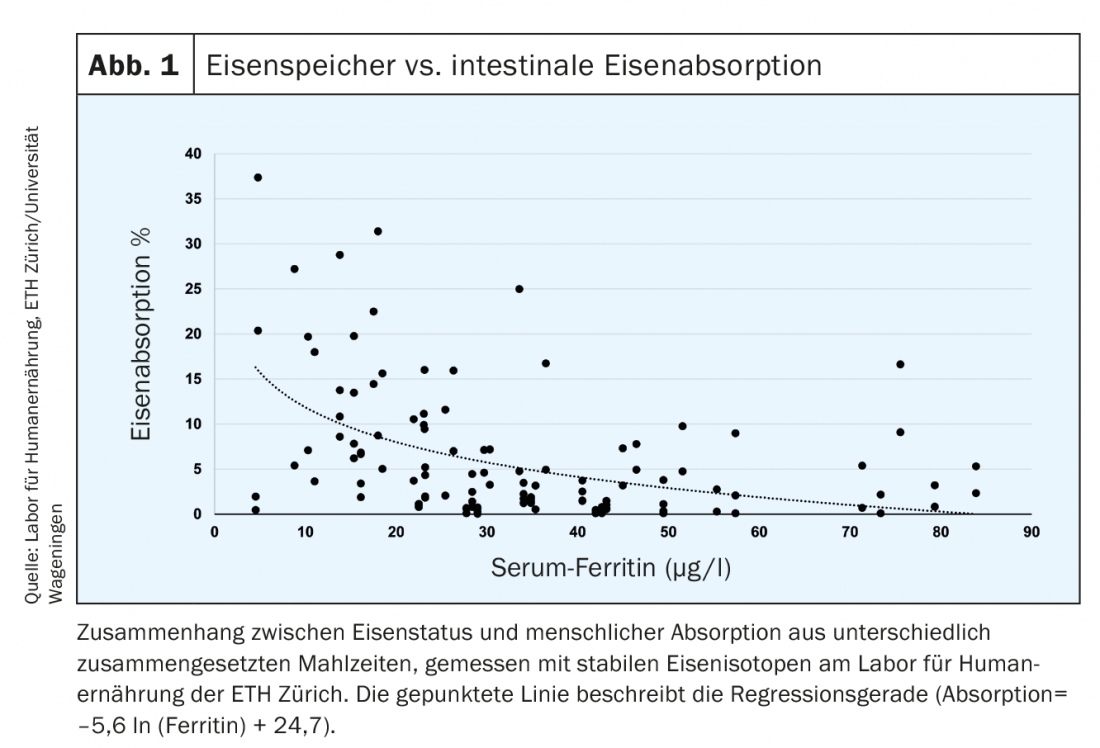
In addition, iron absorption measured with radioactive or stable iron isotopes is increased in patients with iron deficiency (Fig. 1) [6]. In healthy individuals, serum ferritin (SF) thresholds are linearly related to iron stores. 1 µg/l SF corresponds to about 120 µg iron stores/kg body weight or about 8-10 mg iron stores [7]. The defined threshold of 15 µg/l indicates the absence of detectable iron in bone marrow and reflects empty stores. Thus, a further decrease of the value below 15 µg/l has no quantitative significance. Biochemically, SF is the intracellular storage molecule of iron and reflects the accumulation of intracellular ferritin in macrophages and hepatocytes. In healthy patients, SF thus reflects iron stores in the liver. In defective erythropoiesis (thalassemia), there is an accumulation of intercellular iron in macrophages, which also leads to an increase in ferritin. During infection or inflammation, hepcidin levels increase. Cellular iron is only exported in a reduced form and must be stored cellularly (in ferritin molecules). This may result in a “false” elevation of ferritin, which in this case does not indicate increased iron stores.
In patients with infectious or inflammatory diseases, another marker should therefore be determined: the soluble transferrin receptor in serum (sTfR), which is proportional to the iron requirement of the cells. Practically, this value reflects the “starvation” of bone marrow cells for iron. The quotient of SF and sTfR has been shown to be highly predictive [8]. Unfortunately, the very useful marker sTfR is not well standardized; different methods define different thresholds for iron deficiency.
Transferrin saturation is another useful marker that represents the current availability of systemic iron and is decreased during deficiency. This marker may provide evidence of functional iron deficiency, particularly in the context of increased SF due to infection or inflammation. A review of established and experimental biomarkers for iron is provided by Lynch et al. [8].
Prevention through proper nutrition
Dietary iron bioavailability is defined by both its iron content and composition [9]. Medium to high levels of iron are found in legumes, meat, eggs, whole grains, fortified foods, nuts, and seeds. Hemiron is absorbed in increased amounts (intact) (20-50%). Absorption is less susceptible to iron absorption inhibitors and promoters and is less affected by personal iron status. Non-heme iron, on the other hand, is more sensitive to diet composition and iron status, resulting in high variability in iron absorption (2-50%). Despite the small amount of heme iron consumed in the diet, it represents a high percentage of dietary iron intake because it is more bioavailable.
Various components define the absorption of iron in the small intestine, which is also influenced by solubility: Muscle meat or ascorbic acid (vitamin C) have a promoting effect. However, cereals and whole grain products, which are actually rich in iron, contain phytic acid, which reduces the percentage absorption. Polyphenols and tannins in tea and coffee have a similar effect. Ascorbic acid in fruits and vegetables counteracts inhibition. Combinations of iron-rich and vitamin C-rich foods are particularly recommended (overview 1). A varied diet with plenty of ascorbic acid, muscle meat or fish shows an absorption of 15-17% [10].

Oral and intravenous supplementation
Although diet optimization can be effective for primary prevention of iron deficiency, supplementation is recommended in cases of proven iron deficiency to expeditiously correct anemia, reverse iron deficiency in tissues, and replenish iron stores.
Iron supplements are effective but can also cause dose-related side effects. In a study of elderly patients, either 15, 50, or 150 mg Fe was administered over three months. While efficacy was similar at the end of the study, the groups receiving 50 and 150 mg Fe showed more side effects [11].
Iron increases hepcidin concentration for several hours. At doses of ≥60 mg Fe, hepcidin increases in a dose-dependent manner after administration of an iron supplement and remains elevated for up to 24 hours. This increase is associated with a decrease in absorption of about 35%. For this reason, iron supplements can be administered every other day to increase absorption efficiency while maintaining the same dose [12]. Furthermore, it has been shown that splitting the dosage within the same day (e.g., 2× 60 mg Fe instead of 1× 120 mg Fe) has no added value in terms of increased absorption [13].
Doses <60 mg Fe are likely to have a smaller effect on hepcidin. Furthermore, smaller doses can not only effectively prevent iron deficiency, but also have fewer side effects [14].
It should be noted that although oral supplementation in smaller doses increases the percentage absorption, the total amount of iron absorbed decreases. Thus, iron status must also be considered when recommending alternate iron administration. Possible side effects must be weighed against the effect to be achieved.
Intravenous iron is recommended as first-line therapy for severe, symptomatic iron deficiency with anemia [1]. This is generally well tolerated and rarely has side effects. Any negative effects on the intestinal ecosystem, e.g. in pre-stressed patients, are bypassed. In Switzerland, however, the more frequent use of intravenous therapies also evoked criticism, mainly because of the higher costs [15]. The Federal Office of Public Health has now initiated a “Health Technology Assessment”.
Therapy for iron deficiency and iron deficiency anemia should be personalized. Choosing the “right” therapy should include possible causes, degree of iron deficiency, comorbidities, duration of deficiency, and patient preferences. In addition to clinical/physiological reasons, diet and lifestyle (exercise, contraception) play an important role in preventing deficiencies or maintaining a healthy iron status after therapy in otherwise healthy patients.
Take-Home Messages
- To prevent iron deficiency, a varied diet rich in iron and with high iron bioavailability should be recommended.
- With oral supplementation, low doses (between 30 and 60 mg Fe/day) are better absorbed on a percentage basis and are very likely to cause fewer side effects.
- Iron supplementation therapy should be individualized as much as possible. Oral therapy on alternate days should be recommended (e.g., if iron deficiency is not severe: 60 mg/day, every other day).
Literature:
- Camaschella C: Iron deficiency. Blood 2019; 133(1): 30-39.
- Zimmermann MB, Hurrell RF: Nutritional iron deficiency. Lancet 2007; 370(9586): 511-520.
- Milman N, et al: Iron status in pregnant women and women of reproductive age in Europe. Am J Clin Nutr 2017; 106(Suppl 6): 1655S-1662S.
- Van der Merwe LF, Eussen SR: Iron status of young children in Europe. Am J Clin Nutr 2017; 106(Suppl 6): 1663S-1671S.
- Hentze MW, et al: Two to tango: regulation of Mammalian iron metabolism. Cell 2010; 142(1): 24-38.
- Daru J, et al: Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr 2017; 106(Suppl 6): 1634S-1639S.
- Cook JD: Diagnosis and management of iron-deficiency anemia. Best Pract Res Clin Haematol 2005; 18(2): 319-332.
- Lynch S, et al: Biomarkers of Nutrition for Development (BOND)-Iron Review. J Nutr 2018; 148(Suppl 1): 1001S-1067S.
- Hurrell R, Egli I: Iron bioavailability and dietary reference values. Am J Clin Nutr 2010; 91(5): 1461s-1467s.
- WHO/FAO: Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation. Geneva: WHO/FAO 2004.
- Rimon E, et al: Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. American J Med 2005; 118(10): 1142-1147.
- Moretti D, et al: Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015; 126(17): 1981-1989.
- Stoffel NU, et al: Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol 2017; 4(11): e524-e533.
- Bialkowski W, et al: Estimates of total body iron indicate 19 mg and 38 mg oral iron are equivalent for the mitigation of iron deficiency in individuals experiencing repeated phlebotomy. Am J Hematol 2017; 92(9): 851-857.
- Giger M, Achermann R: [Iron substitution in outpatients in Switzerland: Increase of costs associated with intravenous administration]. Z Evid Fortibld Qual Gesundhwes 2013; 107(4-5): 320-326.
HAUSARZT PRAXIS 2019; 14(2): 15-18
CARDIOVASC 2020; 19(3): 6-9