Oral anticoagulation with the non-vitamin K-dependent oral anticoagulants (NOAKs) in patients with atrial fibrillation is a success story. Since the introduction of these substances in Switzerland almost 10 years ago, they have become the preferred therapy for stroke prevention. What is the experience in practice?
Oral anticoagulation with the non-vitamin K-dependent oral anticoagulants (NOAKs) in patients with AF is a success story. Since the introduction of these substances in Switzerland almost 10 years ago, they have established themselves as the preferred therapy in stroke prevention; more recent data from large registries, which are now available, can also confirm the findings from the pivotal studies in patients in everyday life. In this short review, we summarize some new and relevant aspects for practice.
New ESC Guidelines on Atrial Fibrillation
At this year’s Congress of the European Society of Cardiology, which was conducted virtually because of the COVID-19 pandemic, the adapted guideline recommendation for the management of AF was presented and simultaneously published in the European Heart Journal [1]. As an important innovation, the focus is on “simplified” adaptation in the diagnosis and management of this cardiac arrhythmia; the simple mnemonic is “CC to ABC” (Tab. 1) . At the beginning, AF must be correctly diagnosed (Confirm AF) and then an objective characterization of how advanced the problem is (Characterize AF). For the latter, the “4S” apply: assessment of stroke risk, symptom severity, frequency/severity of AF, and finally analysis of AF substrate (morphological changes that have already taken place in the atrium). Once this is done, therapy is initiated. Avoid stroke is of particular importance, but better symptom control and treatment of comorbidities (Comorbidities and cardiovascular risk factors) are also essential factors in the integrative treatment of AF patients. Especially the last aspect should not be underestimated; weight reduction is not only quoad vitam but also crucial with regard to rhythm control in atrial fibrillation [2]. Very similarly, other cardiovascular risk factors include arterial hypertension [3], diabetes mellitus [4], and obstructive sleep apnea syndrome [5]. All of these comorbidities must be addressed if atrial fibrillation is to be treated comprehensively and appropriately for the patient.
Diagnosis of atrial fibrillation
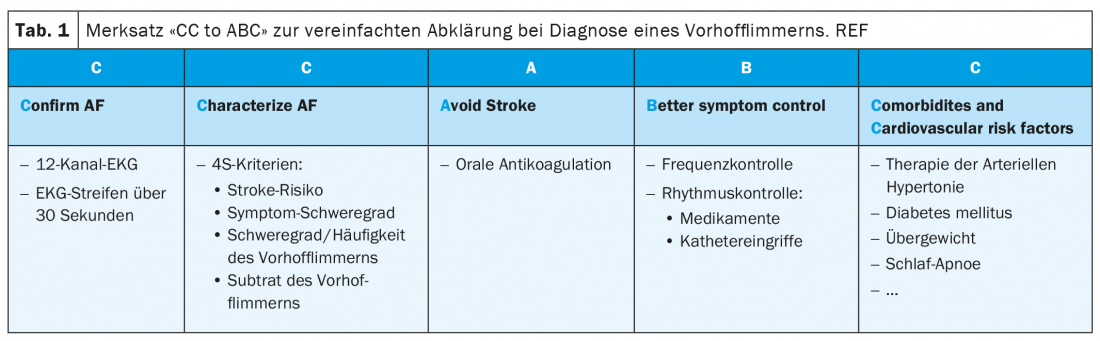
According to the new guidelines, atrial fibrillation is defined as supraventricular arrhythmia with uncoordinated atrial electrical activation and thus insufficient atrial contraction. It is important to distinguish clinical atrial fibrillation from subclinical atrial fibrillation and so-called atrial high rate episodes (AHRE). Clinical atrial fibrillation is present, regardless of the presence of symptoms, if it is recorded on a 12-lead ECG or documented for at least 30 seconds on a plain ECG recording (tab. 2) . Nowadays, more modern diagnostic technologies such as mobile phones and/or specialized watches are used for this ECG recording in addition to the classic long-term Holter examinations. Indeed, two recently published large studies in a healthy population of several hundred thousand people with a mobile ECG (either via a cell phone or a watch) demonstrated that 0.23-0.5% of this population had episodes of an irregular pulse [6,7]. Subsequent cardiac workup confirmed the presence of AF in 84 and 87% of these individuals. We speak of subclinical AF when an automatic recording – e.g., using a pacemaker or smartwatch – suggests AF in an asymptomatic patient, but this cannot be confirmed in a standard ECG [1]. Atrial high rate episodes are mentioned in the guidelines as a special form of subclinical AF. These correspond to paroxysmal atrial tachycardias documented by implanted pacemaker or defibrillator. In all cases, these are defined as tachycardias with an atrial rate greater than 175/min and a minimum duration of 5 minutes. If such episodes are found and documented on an ECG during the course and interpreted as atrial fibrillation, the diagnosis of clinical atrial fibrillation can be made. The management of patients with subclinical atrial fibrillation, both in terms of OAK and possible therapy of the arrhythmia, is currently not definitively clarified. Oral anticoagulation is usually recommended for high stroke risk and frequent/longer-lasting episodes (e.g., >24 hours); however, it is not recommended for low or intermediate risk with rather few episodes (<6 minutes). In the large gray area in between, ongoing studies will provide clarity in the further course [8,9].
Indication for oral anticoagulation in atrial fibrillation.
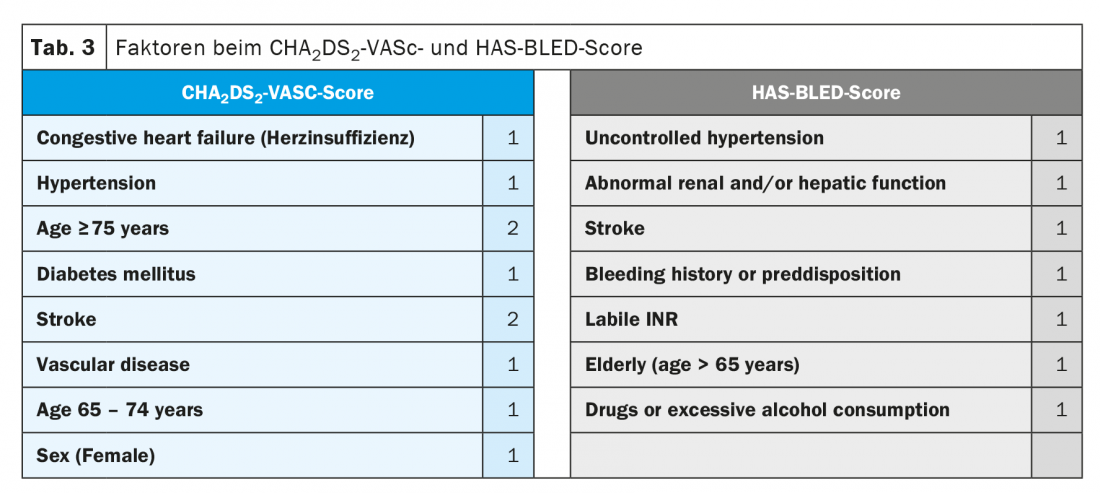
The need for blood thinning in patients with clinical AF is based on risk, calculated using the CHA2DS2-VASC score (Table 3). Importantly, the duration and frequency of AF are not included in the CHA2DS2-VASC score. Although registry data confirm that the risk of thromboembolic events increases with the burden of AF (AHRE/subclinical AF < paroxysmal AF < permanent AF) [10–12], the risk factors described in the CHA2DS2-VASC score alone are decisive for the indication of oral anticoagulation [1]. With a CHA2DS2-VASC score of ≥2 in men or ≥3 in women, oral anticoagulation is indicated, preferably by means of a NOAK (class I recommendation, “level of evidence” A). At a CHA2DS2-VASC score of 0 in men or 1 in women, no blood thinning should be performed, and at a score of 1 (or 2 in women), the guidelines recommend oral anticoagulation with a class IIa recommendation (level of evidence B) [1,13]. New in the current guidelines, the assessment of bleeding risk using the HAS-BLED score has become more important (Table 3) [1]. Importantly, the HAS-BLED score is not used to withhold oral anticoagulation (because it has never been studied for this purpose in a randomized trial), but rather to identify and treat modifiable bleeding risks (for example, inadequately controlled blood pressure, difficult-to-treat patients on vitamin K antagonists) in cases of high bleeding risk (HAS-BLED score ≥3), or to monitor such patients even more regularly, depending on the clinical setting, in order to be able to respond to potential bleeding problems at an early stage [1].
NOAKs in atrial fibrillation and coronary artery disease.
The coexistence of coronary artery disease (CAD) and AF is common and is associated with an increased risk of cardiovascular events. Two different scenarios have to be distinguished: Patients with an acute coronary syndrome or coronary intervention and those with a chronic (formerly called “stable”) coronary situation.
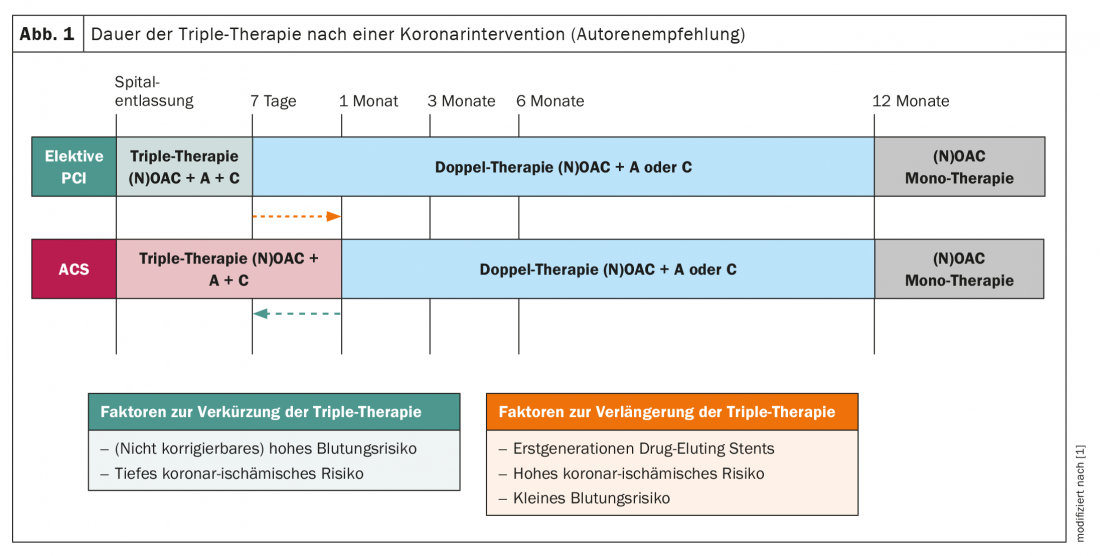
Patients with atrial fibrillation and an acute coronary syndrome or requiring coronary intervention: In the past, patients with atrial fibrillation who experience an acute coronary syndrome or require elective coronary intervention have traditionally been treated with vitamin K antagonist-based triple anticoagulation, often for up to one year. The WOEST study had shown in the course that in this case the harm (bleeding events) is often significantly higher than the incremental benefit (protection against recurrence of thromboembolic events) compared with dual anticoagulation (warfarin and only one antiplatelet agent); this is especially true if the triple therapy is performed over 12 months [14]. Therefore, this long period of more intensive anticoagulation was moved away from some time ago; however, the role of NOAKs in this clinical situation was unclear until recently. Randomized trials are now available for all four agents that have examined the individual NOAKs, including duration of therapy, in this scenario (PIONEER AF-PCI for rivaroxaban [15], RE-DUAL PCI for dabigatran [16], ENTRUST AF-PCI for edoxaban [17], and AUGUSTUS for apixaban [18]). All four studies showed that the incidence of relevant bleeding was significantly lower under a combination of a NOAK with an antiplatelet agent than compared with a triple combination with a vitamin K antagonist, although an effect on all-cause mortality (in contrast to the WOEST study) was not detectable. These data show that the combination of antiplatelet agents with a NOAK is safe in patients with AF and coronary intervention/ACS (Fig. 1) . At the same time, however, it must be mentioned that a consistent signal for a possibly slightly increased rate of myocardial infarction and stent thrombosis presented itself across the studies [19]. Regarding the duration of triple anticoagulation, the new ESC guidelines recommend that after ACS or after an elective intervention, it should be performed for <7 days and then switched to dual therapy (NOAK and usually clopidogrel). However, in the case of increased coronary ischemic risk, this may be prolonged to up to 1 month, which seems reasonable in view of the aforementioned signal for discrete increased risk of myocardial infarction in such pa-tients. Subsequently, dual therapy should be continued thereafter in both clinical scenarios up to and including month 12 (month 6 if bleeding risk is high) (Fig. 1).
Patients with atrial fibrillation and chronic coronary artery disease: Since the ESC guidelines of 2010, based on older studies in this setting, it is recommended to treat patients with chronic CAD and VCF with vitamin K antagonists alone without the addition of aspirin. The pivotal trials of NOAKs for patients with AF have included a relevant proportion of CHD patients. This population did not behave differently from the other study participants, leading to the conclusion that NOAKs can also be given as monotherapy in chronic CHD. In the specifically designed AFIRE trial, 2236 chronic CHD patients with AF in Japan were enrolled and randomized to rivaroxaban (at the Japanese approved dose of 15 mg) plus aspirin or rivaroxaban monotherapy [20]. As expected, monotherapy was superior to combination therapy in terms of bleeding events (HR 0.59; p=0.01); at the same time, there was no increase in thromboembolic events (on the contrary, the number of these events was even numerically lower by 30%). These data support the recommendation that NOAKs should be used as monotherapy (without combination with an antiplatelet agent) in patients with chronic CHD and AF.
Excursus: Anticoagulation in patients with chronic coronary cardiopathy WITHOUT atrial fibrillation.
Patients with chronic CHD without VHF have been treated with aspirin for secondary prophylaxis [21]. Contrary to the previous designation, however, these patients are by no means considered “stable” but continue to have a relevant risk of cardiovascular events as well as higher all-cause mortality. To reduce this rate, the COMPASS trial investigated whether simultaneous inhibition of platelets (by aspirin) and the plasmatic coagulation cascade-the latter using very low-dose rivaroxaban of 2× 2.5 mg per day-showed benefit [22]. In the nearly 24 000 patients included, combination therapy showed a significant 24% reduction in the primary endpoint (stroke, myocardial infarction, cardiovascular death) compared with aspirin alone. Due to the pronounced benefit, the study had to be terminated early one year before the planned end. Although the incidence of hemorrhage was increased by 70% with the combination, there was no difference in clinically relevant major hemorrhage and feared intracranial hemorrhage. This indicates that the majority of bleeding events were not clinically dangerous, in contrast to the ischemic events that were prevented. To compare like with like, a so-called net clinical benefit was predefined in the COMPASS study. This summarizes the most severe thromboembolic and bleeding events. The event rate was 20% lower with combination therapy than with monotherapy. Furthermore, it is important to note that this net clinical benefit increases over time, as bleeding events occurred primarily at baseline – but the risk for ischemic events increases steadily over the years [23]. Finally, it should be mentioned that all-cause mortality was lower in the combination therapy compared with aspirin monotherapy (even though the study was no longer powered for this because of the early termination) [22]. These data have now also found their way into the new ESC recommendations on secondary prophylaxis in chronic CHD, where the combination of aspirin with rivaroxaban 2× 2.5 mg may be considered as a new therapeutic option [24].
Take-Home Messages
- The non-vitamin K-dependent oral anticoagulants are now the gold standard in stroke prevention in atrial fibrillation.
- Fully comprehensive therapy for atrial fibrillation includes anticoagulation and rhythm/frequency control as well as therapy for cardiovascular risk factors.
- Modern devices such as cell phones and digital watches with the appropriate software can also be used to back up the diagnosis.
- NOAKs alone can also be used in patients with coronary cardiopathy in the chronic state.
Literature:
- Hindricks G, Potpara T, Dagres N, et al: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020.
- Pathak RK, Middeldorp ME, Meredith M, et al: Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015;65: 2159-2169.
- Dzeshka MS, Shantsila A, Shantsila E, Lip GYH: Atrial Fibrillation and Hypertension. Hypertension. 2017;70: 854-861.
- Chang SH, Wu LS, Chiou MJ, et al: Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13: 123.
- Li L, Wang ZW, Li J, et al: Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace. 2014;16: 1309-1314.
- Guo Y, Wang H, Zhang H, et al: Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74: 2365-2375.
- Perez MV, Mahaffey KW, Hedlin H, et al: Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381: 1909-1917.
- Lopes RD, Alings M, Connolly SJ, et al: Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189: 137-145.
- Kirchhof P, Blank BF, Calvert M, et al: Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190: 12-18.
- Mahajan R, Perera T, Elliott AD, et al: Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39: 1407-1415.
- Perera KS, Sharma M, Connolly SJ, et al: Stroke type and severity in patients with subclinical atrial fibrillation: An analysis from the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT). Am Heart J. 2018;201: 160-163.
- Ogawa H, An Y, Ikeda S, et al: Progression From Paroxysmal to Sustained Atrial Fibrillation Is Associated With Increased Adverse Events. Stroke. 2018;49: 2301-2308.
- Fauchier L, Clementy N, Bisson A, et al: Should Atrial Fibrillation Patients With Only 1 Nongender-Related CHA2DS2-VASc Risk Factor Be Anticoagulated? Stroke. 2016;47: 1831-1836.
- Dewilde WJ, Oirbans T, Verheugt FW, et al: Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381: 1107-1115.
- Gibson CM, Mehran R, Bode C, et al: Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med. 2016;375: 2423-2434.
- Cannon CP, Bhatt DL, Oldgren J, et al: Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med. 2017;377: 1513-1524.
- Vranckx P, Valgimigli M, Eckardt L, et al: Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394: 1335-1343.
- Lopes RD, Heizer G, Aronson R, et al: Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380: 1509-1524.
- Gargiulo G, Goette A, Tijssen J, et al: Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40: 3757-3767.
- Yasuda S, Kaikita K, Akao M, et al: Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N Engl J Med. 2019;381: 1103-1113.
- Antithrombotic Trialists C, Baigent C, Blackwell L, et al: Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373: 1849-1860.
- Eikelboom JW, Connolly SJ, Bosch J, et al: Rivaroxaban with or without aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377: 1319-1330.
- Steffel J, Eikelboom JW, Anand SS, et al: The COMPASS Trial: Net Clinical Benefit of Low-Dose Rivaroxaban Plus Aspirin as Compared With Aspirin in Patients With Chronic Vascular Disease. Circulation. 2020;142: 40-48.
- Knuuti J, Wijns W, Saraste A, et al: 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41: 407-477.
- Steffel, et al: European Heart Journal 2018; 39: 1330-1393.
CARDIOVASC 2020: 19(4): 6-10