The risk of ischemic stroke is greatly increased in patients with renal insufficiency and atrial fibrillation. In stages II and III renal failure, DOAKs are a good alternative to vitamin K antagonists; with a consistent reduction in systemic embolism and ischemic stroke (dabigatran only), fewer bleeding complications occur with DOAKs. In stage III, dose reduction is recommended for all DOAKs. In stages IV and V renal failure, bleeding complications are an important risk. Apixaban, rivaroxaban, and edoxaban are approved for stage IV at reduced doses, but because of the lack of data, readjustment to these agents cannot be recommended. For stage V renal failure, there is a contraindication to all DOAKs and to vitamin K antagonists. However, if anticoagulation is considered, it must be weighed against the individual risk of stroke and bleeding.
Chronic renal insufficiency (CNI) is an irreversible renal impairment in which the filtration function of the kidney is reduced and breakdown products of protein metabolism cannot be adequately eliminated. Toxic substances such as creatinine, urea and uric acid accumulate in high concentrations in the blood and a dysbalance of the water and electrolyte balance occurs. As early as age 30 years, the prevalence of CNI is 7.2% [1]. Above the age of 70 years, the prevalence increases to approximately 37.8%, becoming a global health problem. CNI is defined by reduction in glomerular filtration rate (GFR) and albuminuria and proteinuria [2].
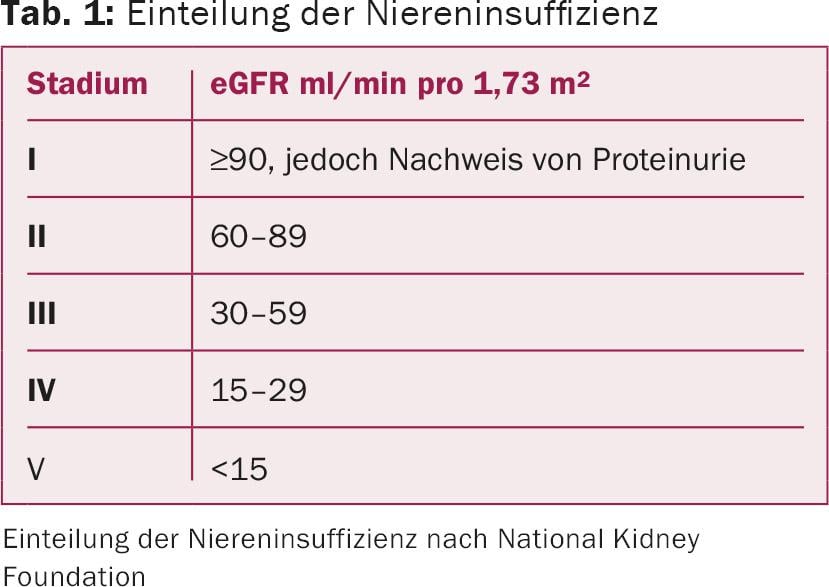
In general, it should be remembered that different methods exist for GFR determination. For risk assessment, the stages of CNI are classified into five categories according to GFR and proteinuria (Table 1) [2]. Determination of physiological GFR via 24-hour urinalysis is accurate but not particularly practical. Therefore, the calculated or estimated GFR is used in clinical practice (eGFR calculated by Cockroft-Gault, MDRD study, or CKD-EPI) [2], which may differ depending on the calculation method due to the different calculation formulas, especially in the higher stages of renal insufficiency.
Non-valvular atrial fibrillation (AF) is an intermittent or permanent cardiac arrhythmia with disordered atrial activity that does not originate from the mitral valve. Based on the NYHA classification for heart failure, the staging is divided into four grades according to the symptoms and impairment of the patient by the VHF (Table 2) . VCF has a prevalence of approximately 1%, divided into 0.5% of the population between 50 and 59 years of age and up to 18% in those over 85 years of age [3].

Relationship between renal insufficiency and atrial fibrillation
Some studies have already shown the association between CNI and increased prevalence for VCF. Renal insufficiency is considered another risk factor for VCF, in addition to arterial hypertension [4]. CNI as well as VHF as stand-alone diseases, but especially in combination, are associated with an increased mortality rate [5]. In a publication with 387 patients with VCF, it was shown that eGFR and CHADS2 score are independent predictors of cardiovascular disease and mortality [6]. Thus, VCF and CNI are associated with increased mortality. Furthermore, an increase in prevalence due to the age of the patients, an influence of renal insufficiency on the development of VCF, and an increased mortality rate in patients with CNI and VCF are shown.
Interaction of renal failure, atrial fibrillation, and stroke.
VHF is the most common cause of ischemic stroke. Cardioembolic cerebral ischemia from atrial fibrillation alone causes approximately 20-25% of all strokes [7,8]. In a recent meta-analysis, the incidence of newly detected VCF after a transient ischemic attack (TIA) or stroke was 23.7% [9]. Embolic strokes caused by VCF are not only larger than those caused by microangiopathy or macroangiopathy, they are also associated with more severe neurologic deficits as well as increased mortality of 20-25% in the first 30 days [10]. This risk increases further when CNI is present due to the aforementioned correlations. With decreased eGFR, the risk of cardioembolic stroke increases to 39% [11]. Several studies show that CNI is a cause of VCF, but development of renal failure requiring dialysis is also associated with increased rates of VCF [4,12,13]. A 5-year Danish cohort analysis of patients with nonvalvular VHF and CNI requiring dialysis confirmed that these patients (CHA2DS2-VASc score ≥2) had a 5.5-fold higher risk of ischemic stroke and thromboembolic events [14].
Vitamin K antagonists
Vitamin K antagonists are anticoagulant drugs that inhibit the production of vitamin K-dependent clotting factors (factors II, VII, IX, and X). Examples include warfarin, phenprocoumon and acenocoumarol. The dosage is individual and is adjusted to the current coagulation (INR). The effect usually appears after a few days and lasts up to five days. In renally healthy patients with VHF and a CHA2DS2-VASc score ≥1, treatment with vitamin K antagonists (target INR: 2-3) is considered the most effective therapy for the prophylaxis of ischemic stroke (Figure 1), whereas treatment with platelet function inhibitors cannot be recommended [15].
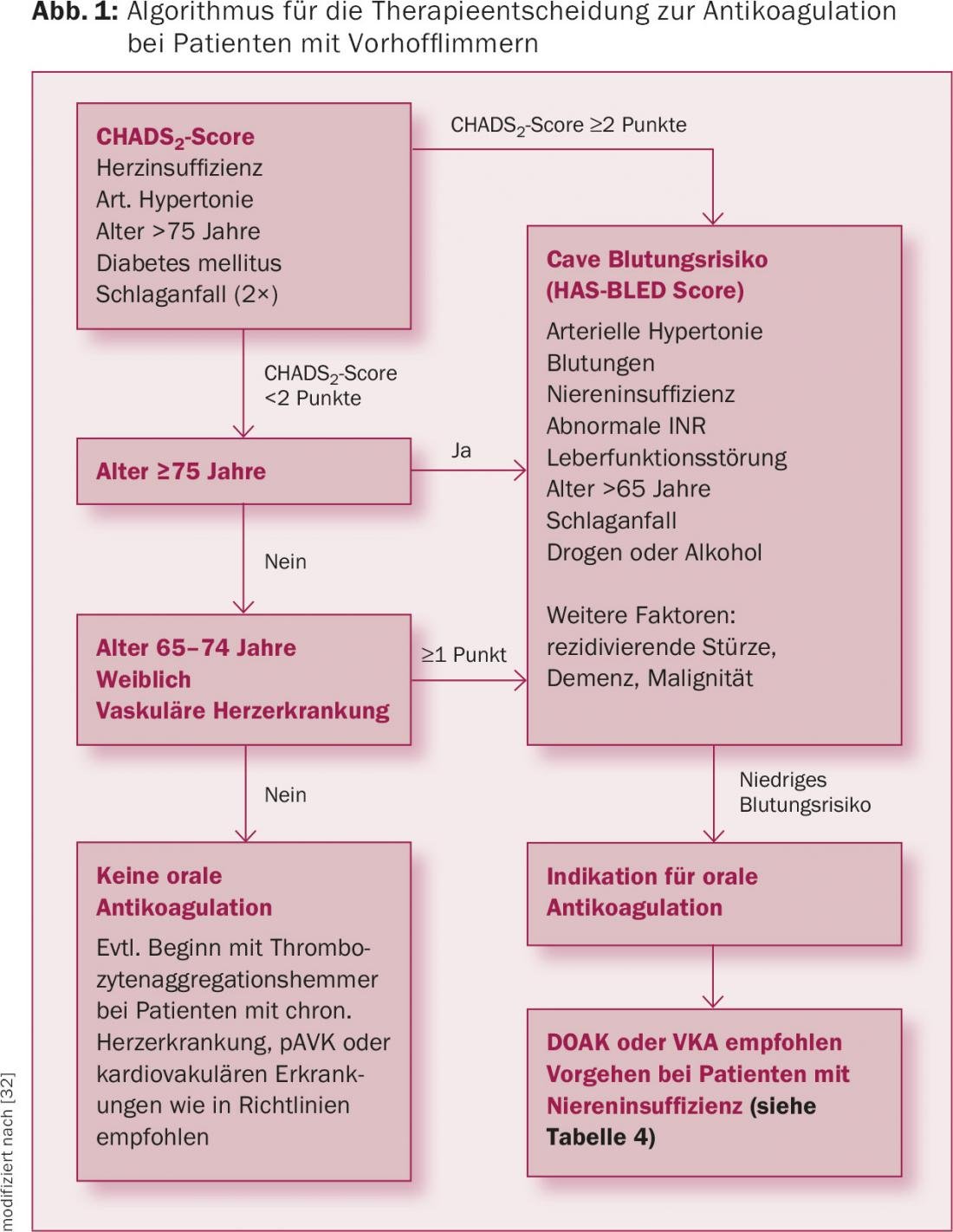
In contrast, the matter is more complex in patients with CNI. Although vitamin K antagonists have also been regularly used in these patients in recent decades, this is contraindicated according to the approval regulations (see Drug Compendium). In addition, in recent years there has been increasing evidence that warfarin, for example, can lead to nephrocalcinosis and thus negatively affect renal function and the outcome itself in the long term [16].
Interestingly, in parallel with the introduction of the new oral anticoagulants (DOAs), the study literature on treatment with vitamin K antagonists in patients with CNI has become more intense in recent years. For example, a prospective Swedish cohort study showed that systemic emboli including stroke were significantly reduced with warfarin treatment in patients with CNI, VCF, and prior myocardial infarction [17]. Interestingly, this was also true for CNI stages III and IV. The previously cited study by Bonde et al. showed that in patients with CNI and CHA2DS2-VASc score ≥2, warfarin treatment reduced the risk of major stroke and bleeding (HR 0.71; 95% CI 0.57-0.88) [14]. Similar results were obtained in a Danish registry study: in 132 372 patients with VCF receiving warfarin therapy, the risk of stroke was reduced by 16% (HR 0.84; 95% CI 0.69-1.01) in patients who had or developed CNI during the course (n=3587) [12]. Interestingly, this effect was also seen in patients with highest grade CNI or dialysis (56% risk reduction; HR 0.44; 95% CI 0.26-0.74). In contrast, a Canadian retrospective cohort study (including meta-analysis) showed no beneficial effect of warfarin on stroke rate or mortality in patients requiring dialysis (adjusted HR 1.14; 95% CI 0.78-1.67) [18]. In this study, a 44% higher risk of bleeding dominated in patients treated with warfarin compared with those not receiving oral anticoagulation (adjusted HR 1.44; 95% CI 1.13-1.85) [18].
New oral anticoagulants
Worldwide, the non-vitamin K antagonists (DOAK; apixaban, dabigatran, edoxaban, and rivaroxaban) are increasingly being used instead of vitamin K antagonists for the prevention of cardiogenic embolic stroke in VCF (Fig. 1). The advantages of DOAKs compared with vitamin K antagonists are their faster onset of action, shorter half-life, and lower potential for interaction with other drugs. Regular determination of coagulation (INR) as with vitamin K antagonists is not necessary. The drugs do not need to be dosed up and have a standard once-dose (rivaroxaban and edoxaban) or twice-dose (apixaban and dabigatran) oral regimen.
In the large randomized trials (ARISTOTLE, ENGAGE AF-TIMI 48, RE-LY, and ROCKET AF), these drugs were shown to be equivalent to warfarin with respect to prophylaxis of new ischemic strokes in patients with VHF [19–22]. Dabigatran was the only substance to show greater efficacy in reducing ischemic strokes at the dose of 150 mg compared with warfarin. It is also significant for clinical practice that DOAKs were able to reduce the incidence rate of intracranial hemorrhage compared with warfarin, sometimes considerably, with a similar general bleeding risk [19–22]. It is encouraging that various options are now available for secondary prophylaxis in patients with stroke and VHF. This results in the need to compare DOAKs with each other, but this seems problematic in terms of efficacy because they have not been tested against each other in randomized trials [23,24]. On the other hand, it seems to make more sense to take a look at the substance properties, e.g. metabolism.
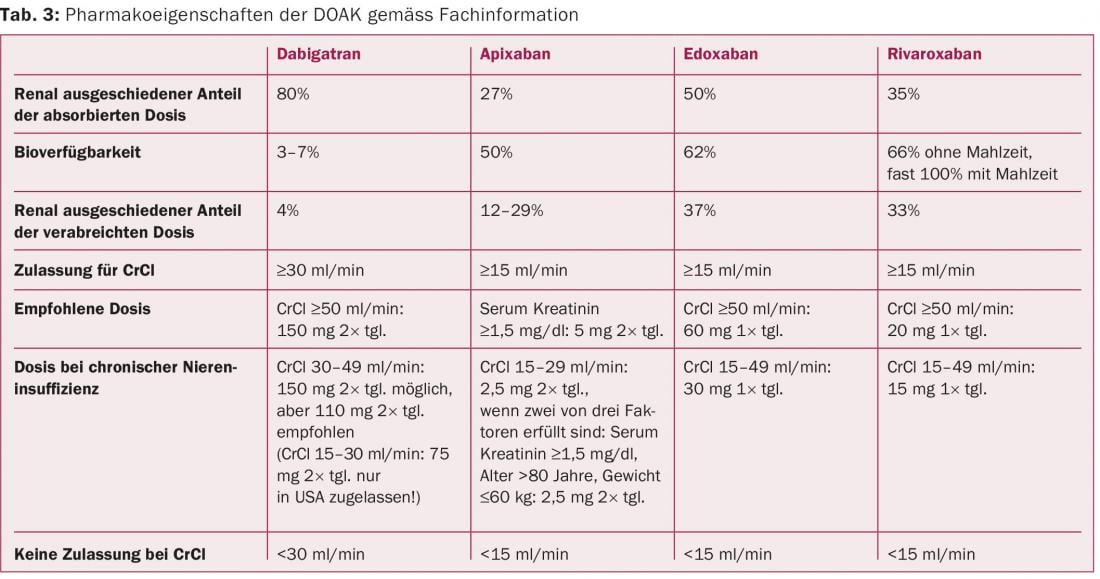
In fact, renal dysfunction is a critical differentiator here because it increases drug levels of DOAK in the blood, which in turn increases the half-life and efficacy of the compounds (Table 3) . This is particularly true for dabigatran, 80% of which is excreted renally and can potentially accumulate in CNI. Dabigatran dosage should therefore be reduced to 2× 110 mg in stage III CNI. Ultimately, this also applies to the less renally excreted factor Xa inhibitors rivaroxaban (reduction to 1× 15 mg), edoxaban (reduction to 1× 30 mg), and apixaban (reduction to 2× 2.5 mg) when at least one other cofactor is present such as age >80 years or weight <60 kg) (Table 3) . All four agents were shown to maintain efficacy after dose reduction in patients with CNI, including the efficacy of dabigatran 150 mg in reducing ischemic stroke). [22,25–27]. Of note, a subgroup analysis of the ARISTOTLE trial suggests that just with impaired renal function, there is a benefit in terms of reduction of bleeding events, while protection against ischemic events was preserved [25]. This was confirmed in a recently published meta-analysis involving 40 145 patients [28].
Procedure for renal insufficiency
Overall, the existing data thus suggest that DOAKs are a good therapeutic alternative to vitamin K antagonists in patients with CNI (Fig. 1). However, the question always arises as to how to proceed here in clinical practice. It seems sensible to make the procedure dependent on the extent of the CNI (Table 4).
In patients with moderate renal insufficiency (stages II and III), both vitamin K antagonists and DOAK may be considered [29]Especially in stage II, DOAK appear to be a good alternative to vitamin K antagonists, as in a recent meta-analysis an advantage was seen for the reduction of systemic embolism as well as ischemic stroke and bleeding was reduced. [30]. In stage III renal failure, dose reduction is recommended for all DOAKs. At reduced doses, these drugs are a good alternative to vitamin K antagonists, because bleeding rates were significantly lower while embolic events were reduced (only dabigatran retains the stroke-reducing effect) [29,30].
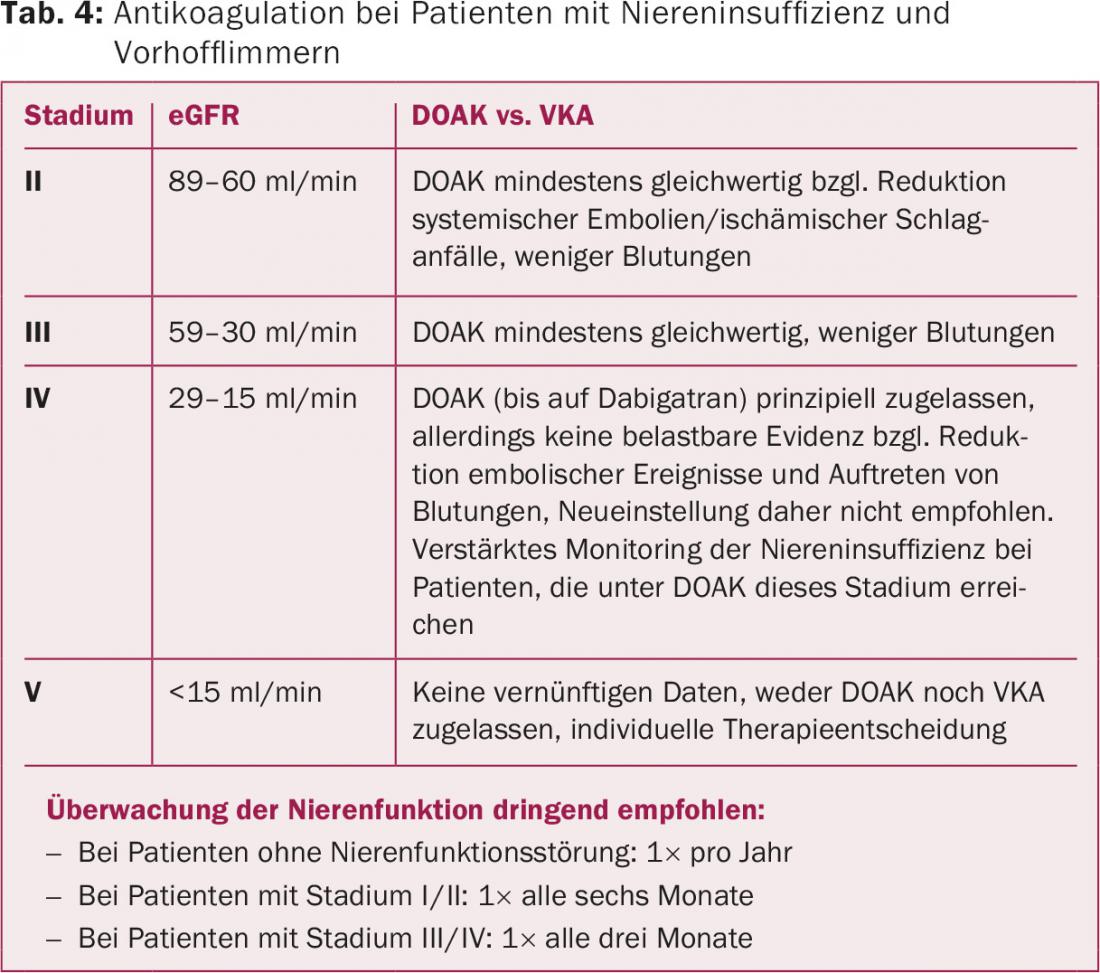
Rivaroxaban, apixaban, and edoxaban are approved in principle at reduced doses for treatment in patients with CNI grade IV. However, it is important to realize that grade IV CNI was a contraindication in the large randomized trials and, ultimately, robust data on efficacy and bleeding complications are not available. Thus, no recommendation can be made for the use of DOAK for this stage of renal failure [29]. In the United States, but not in Europe, a low-dose of dabigatran (2× 75 mg) has been approved in patients with stage IV CNI based on dose and efficacy simulations in patients with CNI.
In stage V renal failure or in patients undergoing hemodialysis, DOAK treatment cannot be recommended, as robust data simply do not exist. Dabigatran and rivaroxaban showed increased bleeding rates compared with warfarin in a recent study in patients requiring dialysis, but the patient numbers were very small and the overall events were few (n=8064 warfarin versus n=281 dabigatran and n=244 rivaroxaban) [31]. Stage V renal insufficiency was a contraindication in the clinical trials and is also designated as such in the product information of the respective preparations. An alternative in terms of an individual therapy decision are vitamin K antagonists, which, however, are also contraindicated in CNI, as already mentioned, and in a recent analysis could not significantly prevent embolic events either, but resulted in more bleeding [18].
Monitoring of kidney function
A significant advantage of DOAK is that close monitoring, such as INR with vitamin K antagonists, is not required. However, due to the described metabolic pathways, regular monitoring of renal function is urgently required. In healthy kidney patients, annual intervals are sufficient. However, patients with CNI should be monitored more closely depending on the stage of CNI, age, concomitant diseases, and the DOAK used, as dabigatran in particular, and to a lesser extent edoxaban, are metabolized in higher proportions by the kidney (Table 4).
Conclusion
Patients with renal insufficiency and atrial fibrillation are among those at highest risk for ischemic stroke. With this in mind, oral anticoagulation should be strongly considered in these patients. In fact, however, depending on the degree of renal insufficiency, anticoagulation can be a real therapeutic challenge, since bleeding complications are a serious risk, especially in stages IV and V. Relatively unproblematic, however, are stages II and III of renal failure, in which therapy with a DOAK is a good alternative to marcoumar or phenprocoumon and, while remaining effective in reducing embolic events, fewer bleeding complications occur.
Stages IV and V of renal failure are more problematic. Although apixaban, rivaroxaban, and also edoxaban are approved for this stage at reduced doses, readjustment to these agents cannot be recommended because of the lack of robust data. However, it is conceivable that patients who are on one of the DOAKs mentioned and reach stage IV in the course of therapy should be kept on it under strict control of renal function in the hope that renal function will improve again in the medium term. For stage V renal failure, there is a contraindication to all DOAK. This also exists for vitamin K antagonists, and the data also do not support a benefit. Ultimately, an individual decision must be made in these patients, weighing the individual risk of stroke as well as concomitant diseases.
Conflicts of interest
The authors declare that they are not guided by any economic interests in the preparation of this article.
let. Wolf-Rüdiger Schäbitz received speaker honoraria from Boehringer Ingelheim, Bayer, Pfizer-BMS and Daiichi. Frédéric Zuhorn has no conflicts of interest.
Literature:
- Zhang QL, Rothenbacher D: Prevalence of chronic kidney disease in population-based studies: systemic review. BMC Public Health 2008; 8: 117.
- National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1-S266.
- Camm AJ, et al: Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369-2429.
- Horio T, et al: Chronic kidney disease as an independent risk factor for new-onset atrial fibrillation in hypertensive patients. J Hypertens 2010 Aug; 28(8): 1738-1744.
- Go AS, et al: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296-1305.
- Nakagawa K, et al: Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol 2011; 107: 912-916.
- Marini C, et al: Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke 2005; 36: 1115-1119.
- Kolominsky-Rabas PL, et al: Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001; 32: 2735-2740.
- Sposato LA, et al: Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systemic review and meta-analysis. Lancet Neurol 2015; 14: 377-387.
- Riva N, Lip GY: A new era for anticoagulation in atrial fibrilation. Which anticoagulant should we choose for long-term prevention of thromboembolic complications in patients with atrial fibrilation? Pol Arch Med Wewn 2012; 122: 45-53.
- Go AS, et al, ATRIA Study Investigators: Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009; 119: 1363-1369.
- Olesen JB, et al: Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Eng J Med 2012; 367: 625-635.
- Bansal N, et al: Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation 2013; 127: 569-574.
- Bonde AN, et al: Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014 Dec 16; 64(23): 2471-2482.
- Diener HC, Weimer C: The new S3 guideline “Stroke Prevention” of the German Society of Neurology and the German Stroke Society. Psychopharmacotherapy 2013; 20: 58-65.
- Brodsky SV, et al: Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2011 Jul; 80(2): 181-189.
- Carrero JJ, et al: Warfarin, Kidney Dysfunction, and Outcomes Following Acute Myocardial Infarction in Patients With Atrial Fibrillation. JAMA 2014; 311(9): 919-928.
- Shah M, et al: Warfarin Use and the Risk for Stroke and Bleeding in Patients With Atrial Fibrillation Undergoing Dialysis. Circulation 2014; 129: 1196-1203.
- Granger CB, et al, ARISTOTLE Committees and Investigators: apixaban versus warfarin in patients with atrial fibrilation. N Engl J Med 2011; 365: 981-992.
- Patel MR, et al, ROCKET AF Investigators: Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883-891.
- Connolly SJ, et al, RE-LY Steering Committee and Investigators: dabigatran versus warfarin in patients with atrial fibrilation. N Engl J Med 2009; 361: 1139-1151.
- Giugliano RP, et al: Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093-2104.
- Lip GY, et al: Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stole prevention in atrial fibrillation. J Am Coll Cardiol 2012; 60: 738-746.
- Ruff CT, et al: Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet 2014; 383: 955-962.
- Hohnloser SH, et al: Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012; 33: 2821-2830.
- Fox KA, et al: Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011; 32: 2387-2394.
- Hijazi Z, et al: Efficacy and Safety of Dabigatran Compared With Warfarin in Relation to Baseline Renal Function in Patients With Atrial Fibrillation. Circulation 2014; 129: 961-970.
- Pathak R, et al: Meta-analysis on risk of bleeding with apixaban in patients with renal impairment. Am J Cardiol 2015 Feb 1; 115(3): 323-327.
- Heidbuchel H, et al.: Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular
- atrial fibrillation. Europace 2015 Oct; 17(10): 1467-1507.
- Sardar P, et al: Novel oral anticoagulants in patients with renal insufficiency: a meta-analysis of randomized trials. Can J Cardiol 2014 Aug; 30(8): 888-897.
- Chan KE, et al: Dabigatran and Rivaroxaban Use in Atrial Fibrillation Patients on Hemodialysis. Circulation 2015; 131: 972-979.
- Reinecke H, Engelbertz C, Schäbitz WR: Preventing stroke in patients with chronic kidney disease and atrial fibrillation: benefit and risks of old and new oral anticoagulants. Stroke 2013 Oct; 44(10): 2935-2941.
CARDIOVASC 2015; 14(6): 16-25