The causes of genetic hair disorders are diverse. Identification of the causative genetic defect plays an important role in understanding the biological mechanisms of disease. In the longer term, new treatment options can be developed based on this, according to the hopes of the human genetics research world. For patients affected by alopecia, molecular genetic clarification allows a conclusive explanation for their hairlessness.
The primary goal of molecular genetic studies is to systematically identify the genes responsible for hairlessness, elucidate their function, and place them in biological networks to better understand the underlying biological mechanisms. “In the medium term, we want to elucidate the physiology of hair growth and, in the long term, find new therapeutic approaches,” said Prof. Regina Betz, MD, University Hospital in Bonn (D) [1]. In the long term, the human geneticist hopes that a better understanding of the causes will improve risk prediction and therapeutic options.
Monogenic forms vs. genetically complex forms of alopecia
“In total, we have three billion letters or nucleotides in the genome,” explains Prof. Betz. The wild type is the non-mutated form of the gene that predominantly occurs in the wild; the rarer mutated variant is called a disease allele or disease variant. It is known that genetic variants can influence interindividual variability, such as appearance, aptitude for something, skin color, hair color or type, but also the tendency to certain diseases. Most diseases are multifactorial, i.e. they result from the interaction of genetic factors and environmental influences. Prof. Betz’s research group investigates the genetic causes of various hair growth disorders: monogenic alopecias, such as hypotrichosis simplex, hereditary congenital hypotrichosis type Marie Unna and alopecia universalis congenitales, as well as the complex forms, such as alopecia areata or androgenetic alopecia. In monogenic or simple inheritance, as is the case in hereditary congenital hypotrichosis type Marie Unna with autosomal dominant inheritance, only one gene is responsible for the expression of the trait. This gene is affected by a pathogenic variant and 50% of it is inherited into the next generation. In autosomal recessive inheritance, such as in alopecia universalis congenitalis, there is a 25% risk of recurrence/disease. Often only one generation is affected, e.g. the parents are carriers and pass the mutation on to the children, who then become ill. In monogenic alopecias, inheritance is autosomal dominant or autosomal recessive. There is no therapy for these rare forms of alopecia, but there is the possibility of molecular genetic diagnostics to determine the cause, but the gene responsible for the disease must already be known. If the diagnosis is definite, single gene sequencing can then be performed; if there are many known genes and the phenotype is unclear, panel diagnostics or, if necessary, an exome can be performed on a research basis [4].
In genetically complex inheritance, such as in alopecia areata (AA), a familial clustering can be observed. These diseases are most likely caused by the presence of mutations in several genes simultaneously in one person. The result is a predisposition to disease, which can turn into a manifest disease under the influence of environmental factors. Psychological components certainly also play a decisive factor in AA. The elucidation of molecular genetic determinants of genetically complex diseases has progressed rapidly in recent years. Methods available today include candidate gene studies, in which it is possible to examine different gene variants; fine mapping studies, in which up to 30 different single nucleotide polymorphisms (SNPs) can be examined in one reaction; and genome-wide association studies, in which correlative relationships between genetic variants in the form of SNPs and specific disease manifestations are examined. In order to be able to uncover overrandom correlations, samples of several thousand subjects are required due to the very large number of SNPs.
Alopecia areata and androgenetic alopecia are most common
Alopecia areata (AA) is the second most common form of hair loss after androgenetic alopecia (AGA). The lifetime risk of developing the disease is estimated to be 1-2% in the general population [1]. AA is thought to have a genetically complex inheritance with multiple disease-contributing genes. The risk of disease for first-degree relatives of an affected person is about 7% for siblings, just under 8% for parents, and just under 6% for children.
All molecular genetic studies performed to date, both candidate gene studies and genome-wide studies, support the hypothesis of an autoimmune genesis of AA [3]. To date, a total of 10 different genomic regions/genes with genome-wide significance have been identified. Of particular note is the HLA region (human leukocyte antigen region), which shows an association in the vast majority of autoimmune diseases. In addition to the HLA region, cytokine-encoding genes, including IL-2/IL-21, IL-2RA, IL-13, as well as other immunoregulatory genes, including CTLA4, show an association with AA [3]. Despite the identified gene loci, there are no definite mutations at these gene loci and consequently no current routine diagnostics to investigate the genetic factors of AA [1].
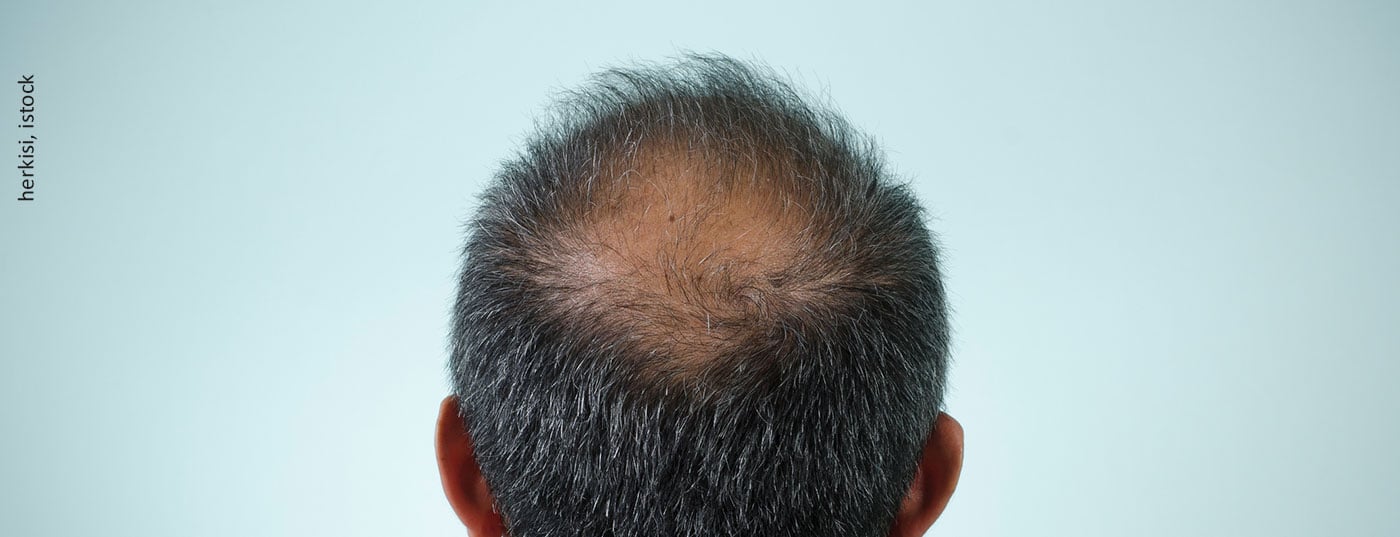
Male androgenetic alopecia (AGA) is also known as hereditary hormonal hair loss and is the most common form of hair loss. AGA is also one of the genetically complex diseases. To date, molecular genetic studies have identified a total of twelve contributing gene loci on chromosomes 2, 3, 5, 7, 12, 17, 18, 20, and X. The only gene that is known to contribute to AGA is the androgen receptor gene located on the X chromosome. Using expression analysis in human hair follicle tissue, additional risk loci on 2q35 (WNT10A), 2q37.3 (HDAC4), 7p21.1 (HDAC9), and 12p12.1 (ITPR2) were also used to suggest possible candidate genes [3]. The finding on 2q35 proved to be particularly promising. Here, a regulatory effect of an AGA risk variant on the expression of the candidate gene WNT10A was demonstrated for the first time. Rather little is known about the genetics of female predisposing hair loss. It appears that the gene loci are very different from those of male AGA.
In both AA and AGA, the genetically complex mode of inheritance can be traced, but no routine diagnosis is yet possible.
Literature:
- Betz R: “Born to be bald – genetics of hair diseases”. DDG Congress, 04/15/2021.
- www.humangenetics.uni-bonn.de/de/forschung/forschungsprojekte/haarlosigkeit-ausfall/androgenetische-frau
- www.humangenetics.uni-bonn.de/de/forschung/forschungsprojekte/haarlosigkeit-ausfall/kreisrunder-haarausfall
- www.humangenetics.uni-bonn.de/de/forschung/forschungsprojekte/haarlosigkeit-ausfall/monogene-allopezien
Further reading:
- Wild type (1st paragraph): https://irihs.ihs.ac.at/id/eprint/5547/1/Lang-et-al-2020-Neue-Anwendungen-der-DNA-Analyse-Technikfolgenabschaetzung-E-Book.pdf
- Diseases are multifactorial (1st paragraph): Ludwig KU, et al: The role of rare variants in common diseases. medgen 2019 – 31: 212-221
- https://doi.org/10.1007/s11825-019-0246-2
- SNPs (1st paragraph): https://toolbox.eupati.eu/glossary/genomweite-assoziationsstudie/?lang=de
HAUSARZT PRAXIS 2021; 16(12): 42-43