In the SEQUENCE study, risankizumab was compared with ustekinumab for the treatment of Crohn’s disease (CD) in patients who had failed anti-TNF therapies. Biomarkers such as fecal calprotectin (FC) and C-reactive protein (CRP) were also measured [1]. In addition to the clinical and endoscopic endpoints, these biomarkers made it possible to objectively assess the course of the disease [1].
CD is extremely stressful for those affected: the underlying inflammation in the gastrointestinal tract can cause permanent intestinal damage and significantly impair quality of life [2, 3]. The two interleukin (IL)-23 and IL-12/-23 inhibitors risankizumab (SKYRIZI®) and ustekinumab, among others, are approved for CD treatment in Switzerland [4, 5]. The two treatment options were compared in the SEQUENCE comparative study published in the New England Journal of Medicine (NEJM) [1]. This is the first head-to-head study in CD to show the superiority of one biologic over another(read the study summary here) [1]. An in-depth analysis
now also shows the better response with risankizumab in several subgroups and in relation to biomarkers [1,6].
Risankizumab also beneficial in subgroup analysis
Over 500 patients with failure to respond to one or more TNF inhibitors were randomized and treated open-label for 48 weeks with either risankizumab (N=255) or ustekinumab (N=265) [1]. In a head-to-head comparison with ustekinumab, risankizumab was superior and all primary and secondary endpoints were met [1]. These included clinical and endoscopic endpoints such as clinical remission (CDAI* < 150) or endoscopic remission at week 48 (SES-CD* ≤ 4 and at least 2 points lower than baseline). In addition, the results in the predefined subgroups were generally consistent with the results of the primary analysis. For example, risankizumab was shown to be advantageous over ustekinumab in terms of achieving endoscopic remission at 48 weeks in patients with different disease durations, severities and localizations of the disease [1].
Significantly greater reduction of biomarkers with risankizumab [6]
A potential limitation of the SEQUENCE study included the open-label study design, which may have influenced the reporting of symptoms. However, in addition to the endoscopic endpoints, which were evaluated centrally without knowledge of the group allocation of the individual patients, the analysis of objective biomarkers such as FC and CRP also underlines the superior efficacy of risankizumab over ustekinumab [1]. FC and CRP are the two most commonly used biomarkers in CD and serve as objective markers of intestinal inflammation [7]. Normalization of FC and CRP is therefore also recommended as a medium-term treatment goal in the STRIDE II guidelines [7]. In the SEQUENCE study, FC and CRP were measured in patients before the start of treatment and at weeks 8, 24 and 48. After both 24 and 48 weeks, the FC level in the risankizumab group had reduced significantly more on average than in the ustekinumab patients (Fig. 1) [6]. The evaluation of the CRP values yielded similar results. Already after 8 weeks, a significantly greater reduction from baseline was seen with risankizumab than with ustekinumab (Fig. 1) [6]. Overall, the reduction in FC and CRP correlated with the clinical and endoscopic remission rates after 48 weeks, in which risankizumab showed superiority over ustekinumab [1].
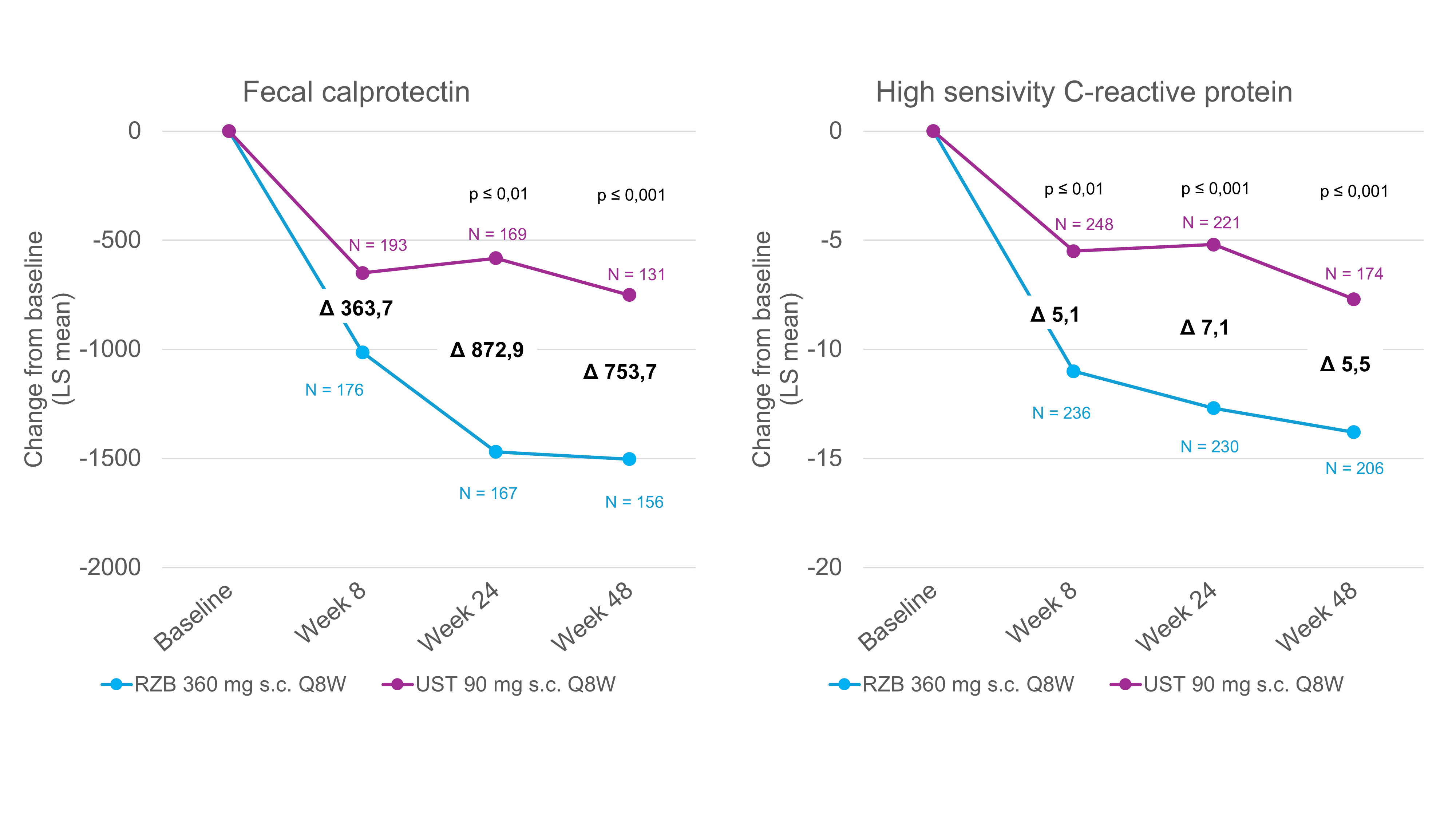
Fig. 1. Risankizumab leads to a significantly greater reduction in biomarkers compared to ustekinumab. LS = least-squares; Q8W = every 8 weeks; RZB = risankizumab; s.c. = subcutaneous; UST = ustekinumab. Post-hoc analysis, all P-values are nominal and not multiplicity controlled. Adapted from [6]
Conclusion
The SEQUENCE study shows that risankizumab is not only clinically and endoscopically superior to ustekinumab in CD treatment, but also enables a faster and greater reduction in the objective biomarkers FC and CRP [1]. The improvement in biomarker values underlines the potential of risankizumab to enable sustained control of disease activity and offer patients a better quality of life in the long term.
* Abbreviations: CDAI = Crohn’s Disease Activity Index; SES-CD = Simple Endoscopic Score for Crohn’s Disease.
Short technical information SKYRIZI®
Report: Dr. sc. Stefanie Jovanovic

This article was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, Cham.
CH-SKZG-240070 10/2024
This article has been released in German.
Literature
1 Peyrin-Biroulet, L., et al, Risankizumab versus ustekinumab for moderate-to-severe Crohn’s disease. N Engl J Med, 2024. 391(3): p. 213-223.
2 Jairath, V. and B.G. Feagan, Global burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol, 2020. 5(1): p. 2-3.
3 The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol, 2020. 5(1): p. 17-30.
4. current expert information on SKYRIZI® (risankizumab) Crohn’s disease at www.swissmedicinfo.ch.
5. current technical information on ustekinumab at www.swissmedicinfo.ch.
6 Dubinsky, M., et al. Risankizumab Versus Ustekinumab for the Achievement of Clinical Remission and Reduction in Inflammatory Biomarkers in Patients With Moderate-To-Severe Crohn’s Disease: Results From the Phase 3B SEQUENCE Trial. Oral presentation 763 at DDW 2024; Washington D.C., USA, May 19-21, 2024.
7 Turner, D., et al, STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology, 2021. 160(5): p. 1570-1583.
The references can be requested by specialists at medinfo.ch@abbvie.com.











