The treatment options for cartilage damage usually allow significant relief of discomfort and restoration of professional and recreational sporting ability. Early treatment is indicated to avoid irreversible changes in joint homeostasis. The analysis of the basic mechanical conditions is crucial for the success of the treatment.
The articular cartilage, especially of the knee joint, is exposed to extraordinarily high stresses at work and in sports. The increasing number of overweight patients also leads to an increased prevalence of articular cartilage damage, so that both specialists and general practitioners are often faced with the question of how to treat damage to the articular cartilage cover in the best possible way. The basic problem of articular cartilage damage is its low capacity for self-healing. The cartilage cells (chondrocytes) embedded in the cartilage matrix lose the ability to divide at a young age, during adolescence – one of the basic requirements for tissue regeneration and thus healing. This circumstance explains why cartilage damage has only a very limited capacity to heal itself, and why in the case of relevant symptoms surgical repair or at least symptom relief by means of conservative therapy must not infrequently be discussed.
The intact articular cartilage cover has the task of absorbing shocks, compensating for inaccuracies in the fit of the joint surfaces and transmitting the forces that occur to the underlying subchondral bone in as attenuated a manner as possible. In this context, the collagen-mediated inherent elasticity of articular cartilage is relatively low compared to its ability to undergo a change in shape by means of controlled uptake and release of fluid. The process of fluid absorption and release is highly complex and is mediated by various macromolecules, some of which have an enormous water-binding capacity. For example, 1 g of hyaluronic acid can bind up to 6 l of water. In addition to hyaluronic acid, probably the best-known macromolecule from the proteoglycan group, chondroitin sulfate, glucosamine sulfate and aggrecans play a significant role in the water balance of the “organ” articular cartilage, which consists of about 80% water.
The chondrocytes, i.e. cartilage cells, are in turn responsible for the demand-oriented production of these proteoglycans. Any disturbance of the synthesis capacity of the chondrocytes inevitably leads to a reduced load-bearing capacity of the tissue and consequently to its demise. This process can be caused by direct force in the case of an accident, chronic overload, such as in the case of overweight, joint instability, altered joint congruency in the case of a partial meniscus removal that has taken place, or a biomechanically relevant axial deviation (knock-kneed or bow-legged).
The process that describes this circumstance is commonly called osteoarthritis. This is not a defined condition of the joint, but rather a gradual and initially unnoticed change at the level of so-called joint homeostasis. This change involves catabolic and chronic inflammatory processes over a variable period of time and eventually leads to disintegration of the articular cartilage, which in turn causes structural changes in the subchondral bone. In conventional radiography, this becomes apparent in the late stages in the form of hypersclerosis. The osteophytes that occur in this context are also a consequence of such an adversely altered joint environment, which allows the formation of ossifications to become visible in part early in the arthrosis process.
These consequences of a disturbed cartilage and joint metabolism, which are only sketched very superficially here, are intended to illustrate how essential it is for the biochemical processes to run harmoniously for the integrity of the articular cartilage. This also shows how deleterious even the smallest deviations from the norm can be for the survival of the affected joint, whether in the metabolic sense, e.g. in the case of a systemic disease (rheumatism, gout, infections) or also in the case of an initially purely mechanical “derailment” of joint homeostasis after e.g. cruciate ligament rupture or meniscus removal.
Maintaining or restoring this joint homeostasis is the goal of nonoperative treatment of articular cartilage damage.
Conservative treatment of articular cartilage damage
Conservative treatment of articular cartilage damage includes a wide range of measures to counteract the process of osteoarthritis development. These include physiotherapeutic and physical measures, which primarily aim to maintain joint mobility and muscular stabilization. Another pillar of conservative treatment is the use of cartilage-robbing substances. An almost unmanageable number of preparations with a wide variety of ingredients are available on the market. Not many of these preparations or compositions have a scientifically proven benefit.
- Glucosamine sulfate and chondroitin sulfate are, as mentioned at the beginning, two of the main components of the cartilage matrix and are responsible for coordinated fluid absorption and release. There are a large number of studies, some of high quality, with large numbers of subjects, which suggest a low to moderate effect of taking these substances, preferably in combination. Thus, taking these substances at a dosage of about 1200 mg/d glucosamine sulfate and 800 mg/d chondroitin sulfate should have a chondroprotective effect and, compared with placebo, result in less pain.
- Another approach to the treatment of cartilage damage and osteoarthritis, or the inflammatory reactions caused by it, is the use of so-called radical scavengers. These are substances that are supposed to help buffer oxygen radical-mediated inflammatory processes and thus indirectly – via alleviating the inflammatory component – have a positive effect on catabolic and pain processes. Established substances are vitamin C, E and trace elements such as selenium, copper and zinc.
The conservative treatment of articular cartilage damage also usually includes the injection of pain-modulating substances into the affected joint. There are three main substances to be mentioned here:
- Corticosteroids
- Hyaluronic acid preparations
- Platelet-rich plasma (PRP) preparations.
Cortisone injections are an effective means of achieving relevant relief of symptoms over a short period of a few weeks. However, an actual treatment of cartilage damage is not possible with this. On the contrary – the mostly crystalline dosage form of injectable corticosteroids has a detrimental effect on the articular cartilage layer, especially with repeated administration.
The therapeutic benefit of hyaluronic acid preparations has been well studied scientifically and is pain and inflammation relief over a period of about half a year. Repeated application is possible, but often exhibits shortened or reduced efficacy compared to first application. In particular, in the case of knee joint osteoarthritis, repeated hyaluronic acid injections have been shown to significantly increase the time interval until the need for prosthesis implantation. In daily practice, these preparations, in addition to alleviating postoperative irritation, are suitable when patients do not yet feel ready for joint replacement and the gain in time with increased quality of life opens up the option of calmly considering the further procedure.
The active principle of PRP preparations, on the one hand, consists in the initiation of a tissue-regenerating cascade by means of the release of so-called growth factors and, on the other hand, in the direct positive influence on prevailing inflammatory processes.
The use of platelet-rich plasma (PRP) has experienced a real hype in recent years. As is so often the case in medicine, the implementation of the treatment method took place before the actual proof of practical benefit.
In the meantime, there is a certain consensus that the injection of PRP, especially in the case of incipient osteoarthritis, provides a significant benefit in comparison to placebo, as well as in comparison to hyaluronic acid, and that this effect can probably be maintained over a longer period of time than with the aforementioned procedures. Potentially, the application of growth factors would be able not only to slow down degenerative processes, but also to partially reverse them, thus contributing to substantial healing. The knowledge about which factors are needed in which concentration at which time to promote such process flows is currently not yet sufficiently available.
Surgical treatment of articular cartilage damage
There are essentially three different procedures for surgical reconstruction of articular cartilage damage:
Bone marrow stimulating procedures
- (Microfracture/AMIC®)
- osteochondral transplantation (mosaicplasty)
- autologous cartilage cell transplantation
The main difference between these treatment methods is the origin of tissue regeneration.
In the most common method, microfracturing and its modification, the AMIC® procedure, tissue regeneration is based on the differentiation of mesenchymal stem cells from the bone marrow into cartilage replacement tissue that is as close to hyaline as possible.
The difference between the two surgical techniques is the use of a collagen membrane in AMIC®, which allows potentially larger defects to be treated than is possible in the case of microfracturing alone (Fig. 1 A-C).
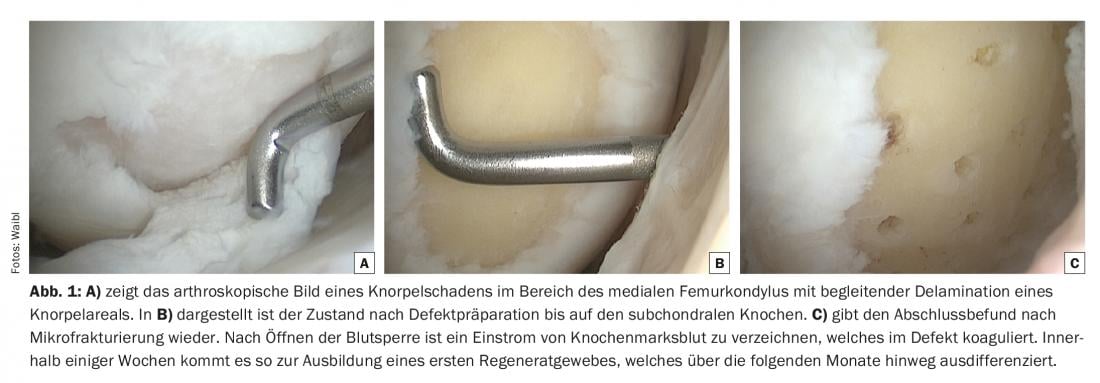
The disadvantage of both procedures is the uncontrollable differentiation of the stem cells, which often only mature into primitive scar tissue or ossify as a final state in the former defect area, with correspondingly disadvantageous biomechanical properties of the tissue regeneration. The clinical results correlate to this trend and, especially in the case of microfracturing, showed a significant decrease in the reduction of discomfort already about one and a half years postoperatively. Within five years of the procedure, surgical revision is expected in a high percentage of these patients.
AMIC® appears to be superior to microfracturing in the medium term over three to five years and to be able to maintain the decent functional results over a longer period of time. In recent years, some modifications of bone marrow stimulating procedures have been developed (e.g. BST-CargelTM), which should help to improve the quality of regenerated tissue. A final assessment of the utility of this procedure has yet to be made. Initial studies of medium-term outcomes demonstrate potential benefits.
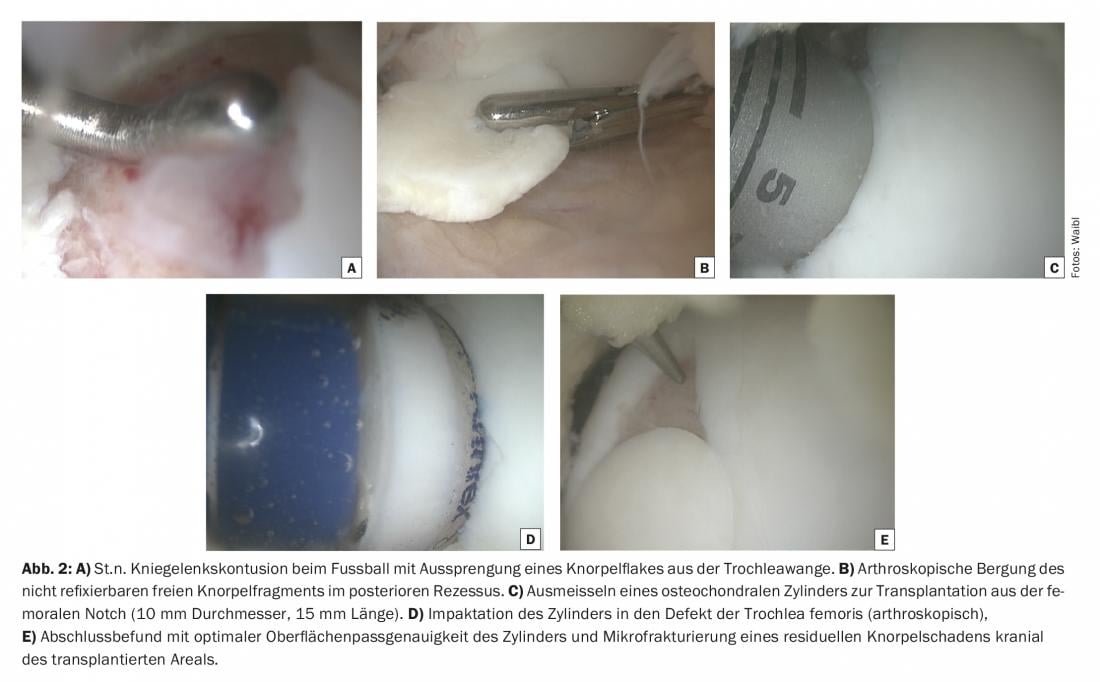
Osteochondral transplantation, also known as mosaicplasty, is based on the transplantation of healthy cartilage-bone tissue from a low-load area of the joint to a high-load area. In the past, this procedure was actually used similar to a mosaic in the sense of transplanting multiple, small-caliber cartilage-bone cylinders even in large defects. In this case, the results were not infrequently disappointing. The practically demanding technique of impaction of several cylinders congruently on the surface was accompanied by a biological limitation of the incorporation of the cylinders, which is why the procedure fell somewhat out of fashion (Fig. 2 A-E). In the meantime, it has become generally accepted that cartilage-bone damage with a defect extent of up to 3 cm2 with one or two cylinders up to about 12 mm diameter can be supplied in a technically and biologically reliable manner. Clinical results support this with very high patient satisfaction, rapid rehabilitation and very satisfactory long-term durability even beyond 10-15 years. Especially in young patients with high athletic demands, osteochondral transplantation is the procedure with the fastest (a good five months) and most reliable (>90%) return to (competitive) sports.
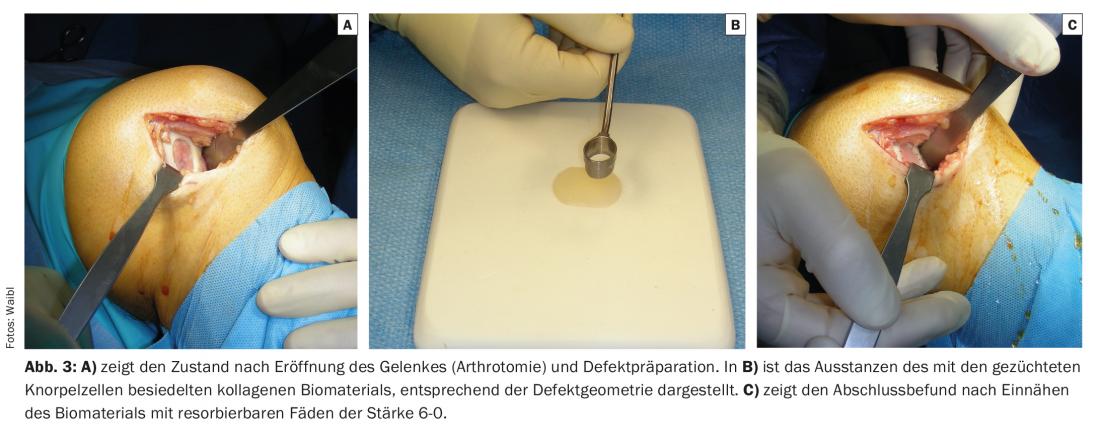
The third surgical procedure that has proven its efficiency in the last 20 years or so is autologous chondrocyte cell or chondrocyte transplantation. This is based on taking a cartilage biopsy as part of an arthroscopic intervention and using an enzymatic and mechanical process to detach the chondrocytes from the cartilage matrix surrounding them, which causes them to enter a state of divisibility. Within a few weeks, a multiplication of cartilage cells takes place in several stages, so that even a large damaged cartilage area (up to >10 cm2) can be covered. After transfer to the defect, the transplanted cartilage cells are able to develop their metabolic activity: They regenerate a repair tissue histologically and biomechanically similar to the native cartilage, which is also able to withstand the high forces in the joint in the long term. (Fig. 3 A-C). The operational, logistical and, above all, financial effort involved in this procedure is comparatively the highest, which explains its limited use and support on the part of insurers. Due to its long-term durability, the procedure has recently been reimbursed by accident and health insurers, taking into account defined indication criteria.
Further reading:
- Wildi L, Flatz A, von Elm E: Is chondroitin effective in osteoarthritis? Praxis 2016; 105(10): 587-588.
- Dhillon MS, et al: PRP in OA knee – update, current confusions and future options. SICOT J 2017; 3: 27.
- Laver L, et al: PRP for Degenerative Cartilage Disease: A Systematic Review of Clinical Studies. Cartilage 2016; 1947603516670709.
- Ulstein S, et al: Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc 2014; 22(6): 1207-1215.
- Pareek A, et al: Osteochondral Autograft Transfer Versus Microfracture in the Knee: A Meta-analysis of Prospective Comparative Studies at Midterm. Arthroscopy 2016; 32(10): 2118-2130.
- Pareek A, et al: Long-Term Outcomes after Autologous Chondrocyte Implantation: A Systematic Review at Mean Follow-Up of 11.4 Years. Cartilage 2016; 7(4): 298-308.
- Volz M, et al: A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop 2017; 41(4): 797-804.
- Krych AJ, et al: Return to sport after the surgical management of articular cartilage lesions in the knee: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 2016 Aug; doi: 10.1007/s00167-016-4262-3 [Epub ahead of print].
HAUSARZT PRAXIS 2017; 12(7): 12-17











