Diabetes is associated with an increased cardiovascular risk, which should be taken into account therapeutically. In 2015, the EMPA-REG OUTCOME study demonstrated positive cardiovascular effects of the SGLT-2 inhibitor empagliflozin. The results of the largest cardiovascular study to date in this field are now available: DECLARE-TIMI 58 investigated the safety and efficacy of dapagliflozin.
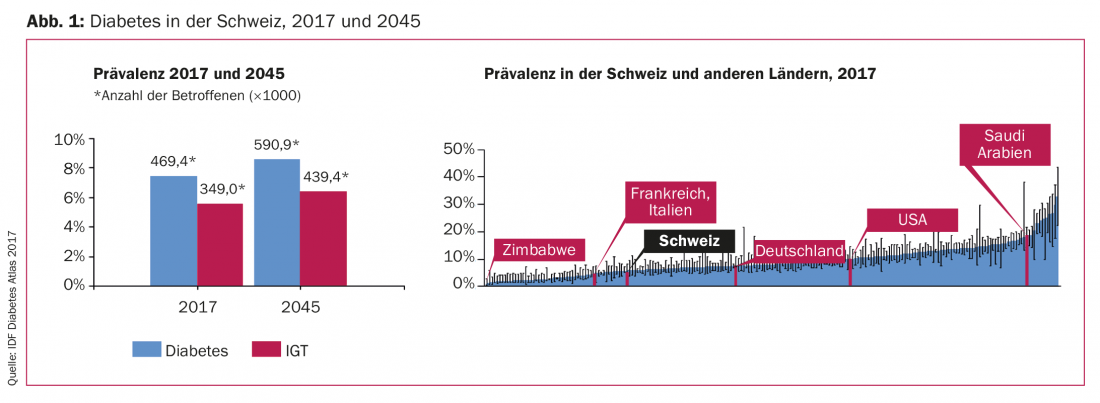
An estimated 451 million people worldwide suffer from diabetes. By 2045, this figure is expected to be around 690 million [1]. This trend also applies to Switzerland, whose diabetes prevalence is in the middle range in a global comparison (Fig. 1). Patients with diabetes have an increased risk of adverse outcome of atherosclerotic cardiovascular disease, heart failure and kidney disease. Therefore, therapeutic measures should not only address diabetes but also reduce cardiovascular risk. In this context, SGLT-2 inhibitors have emerged as a promising new class of antidiabetic agents.
Largest Outcome Study to Date on SGLT-2 Inhibitors
After EMPA-REG OUTCOME (empagliflozin, Boehringer Ingelheim) [2] and CANVAS/CANVAS-R (canagliflozin, Janssen) [3], the results of the largest study in the field of SGLT-2 inhibitors to date are available: DECLARE-TIMI 58 (“Dapagliflozin Effect on Cardiocasvular Events – Thrombolysis in Myocardial Infarction 58”) is a randomized, double-blind, placebo-controlled phase III trial conducted at 882 sites in 33 countries [4]. The objective of the study, which was funded by AstraZeneca, was to demonstrate the safety and efficacy of dapagliflozin.
The study included 17,160 type 2 diabetic patients with cardiovascular risk profiles. Of these, nearly 10,200 were not yet suffering from cardiovascular disease. Study participants received dapagliflozin 10 mg daily vs. placebo in addition to standard therapy over an observation period of 4.2 years.
The primary safety endpoint was major adverse cardiac events (MACE), defined as cardiac-related death, myocardial infarction, or ischemic stroke. The primary efficacy endpoints were MACE, cardiac-related death, and hospitalization for heart failure. Secondary efficacy end points defined were death from any cause and renal events (decrease in eGFR by ≥40% to <60 ml/min per 1.73 m2 body surface area; new-onset end-stage renal disease; death of renal or cardiovascular cause).
Positive effects in heart failure and kidney diseases
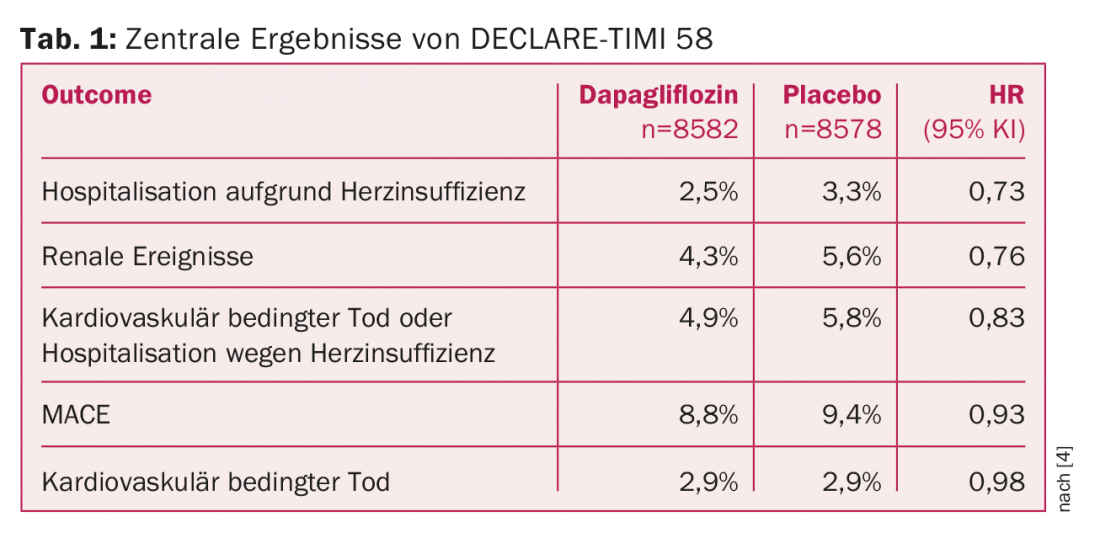
Dapagliflozin was shown to be non-inferior to the placebo control group with respect to the primary safety endpoint (MACE). No significance was present in the reduction of MACE, although a weak trend emerged in this regard in the patient population with existing cardiovascular disease. However, compared with the placebo group, dapagliflozin therapy significantly reduced the incidence of cardiovascular-related death and hospitalization for heart failure. Thus, DECLARE-TIMI 58 confirms the results of EMPA-REG OUTCOME. However, the latter study caused a stir in 2015 by additionally showing a positive effect of empagliflozin also on all-cause mortality.
However, Stephen Wiviott, MD, co-study director and active at Brigham and Women’s Hospital and Harvard Medical School, emphasized that the patient population of DECLARE-TIMI 58 differs from that of previous studies in the field. First, EMPA-REG OUTCOME and CANVAS/CANVAS-R studied fewer patients with shorter follow-up (7000 and 10,000 during three years and 188 weeks, respectively). Second, DECLARE-TIMI includes 58 predominantly “healthier” patients without preexisting cardiovascular disease. For this population, appropriate study data confirming the efficacy of SGLT-2 inhibitors have been lacking.
Dapagliflozin is also effective in renal disease, according to study results. While this was suspected with respect to canagliflozin in CANVAS/CANVAS-R, participants in the DECLARE-TIMI 58 trial showed lower rates of renal disease progression-regardless of the presence of atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease at baseline (Table 1).
What about the side effects?
Various studies with SGLT-2 inhibitors sometimes referred to an increased risk of amputations, diabetic ketoacidosis, stroke, or bone fractures, although reliable data sometimes do not exist here [2,3,5,6].
Although cases of bladder cancer had occurred in previous, smaller trials of dapagliflozin, lower rates were seen in DECLARE-TIMI 58. In contrast, diabetic ketoacidosis occurred more frequently after the use of dapagliflozin compared with the placebo control group. Also elevated were rates of genital infections. There were no differences from placebo treatment with respect to Fournier’s gangrene.
Overall, as in the empagliflozin and canagliflozin trials, there were stronger effects on prevention of heart failure and renal disease outcome than on the incidence of atherosclerotic cardiovascular events. This finding is consistent with the mechanism of action of SGLT-2 inhibitors.
Source: American Heart Association (AHA) Scientific Sessions, November 10-12, 2018, Chicago (USA).
Literature:
- Cho NH, et al: IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018; 138: 271-281.
- Zinman B, et al: Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373 (22): 2117-2128.
- Neal B, et al: Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377(7): 644-657.
- Wiviott SD, et al: Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2018; doi: 10.1056/NEJMoa1812389 [Epub ahead of print].
- Garg SK, et al: Strategy for Mitigating DKA Risk in Patients with Type 1 Diabetes on Adjunctive Treatment with SGLT Inhibitors: A STICH Protocol. Diabetes Technol Ther 2018; 20(9): 571-575.
- Imparialos KP, et al: Stroke paradox with SGLT-2 inhibitors: a play of chance or a viscosity-mediated reality? J Neurol Neurosurg Psychiatry 2017; 88(3): 249-253.
CARDOVASC 2018; 17(6): 34-35 (published 11/28/18, ahead of print).