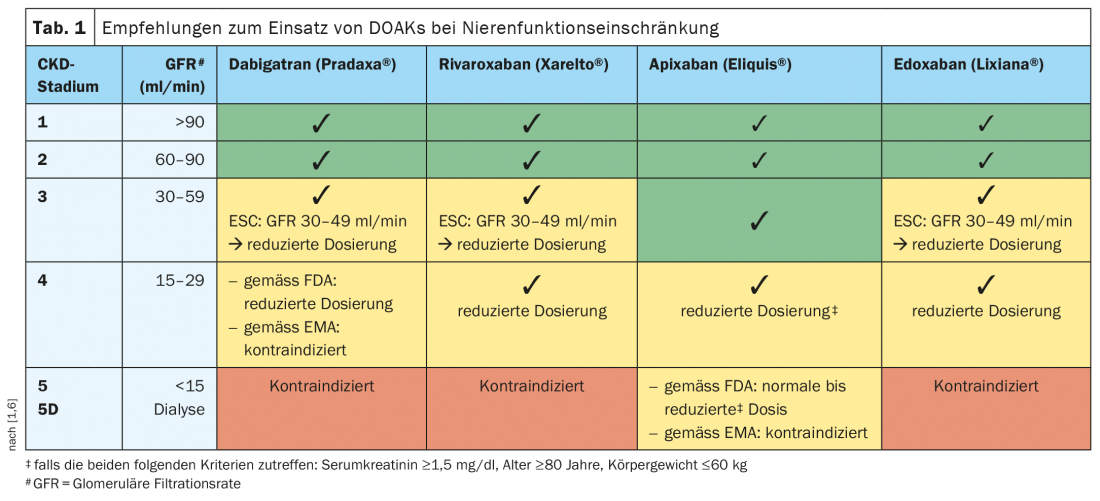
Any anticoagulation is a balancing act between thromboembolism and bleeding risk. This is particularly true in the case of chronic kidney dysfunction (CKD). In mild to moderate renal disease, all currently approved DOAKs in Switzerland can be used in normal doses, whereas in advanced renal insufficiency, the individual risk profile should be taken into account and, if necessary, an adjusted dosage should be used. Caution is advised in CKD stage 5.
Currently, various direct oral anticoagulants (DOAKs) are prescribed in Switzerland for the prevention and treatment of thromboembolic disease. The effect of DOAKs is based on the specific and direct inhibition of activated coagulation factors. With regard to dabigatran, this concerns thrombin; with regard to rivaroxaban, edoxaban and apixaban, activated factor X (Xa). The substances currently approved in Switzerland differ in terms of renal elimination rate. Dabigatran (Pradaxa®) is excreted 80% by the kidney, whereas apixaban (Eliquis®) is excreted only 27%. Edoxaban (Lixiana®) is 50% and rivaroxaban (Xarelto®) is 35% renally eliminated [1,2]. “Up to a GFR of 60 ml/min, all substances can be used,” said Prof. Thomas Fehr, MD, Chief of Internal Medicine, Graubünden Cantonal Hospital, summarizing the current guideline recommendations for a use of the four currently approved DOAKs in mild and moderate renal insufficiency (Tab. 1) [1,3]. Apixaban was even shown to have a significantly greater reduction in bleeding risk compared with vitamin K antagonists above a GFR <50 (>30) ml/min [4,5].
DOAKs in adapted dosage generally applicable up to GFR >15 ml/min
Most dosing recommendations are based on pharmacokinetic calculations rather than clinical studies. Empirical data on anticoagulation with DOAKs or vitamin K antagonists in patients with a CrCl* <25 ml/min are scarce because this patient population was excluded from most RCTs. Only the studies with apixaban included patients with GFR up to 20 ml/min [5]. The current ESC guideline recommendation is that rivaroxaban, edoxaban, and apixaban can be used in adjusted dosing in severe CKD (CrCl 15-30 ml/min) [3]. There is a relative contraindication in patients with a GFR<15 ml/min. Prof. Fehr explains, “There is insufficient evidence to systematically anticoagulate patients on hemodialysis with nonvalvular atrial fibrillation for stroke prevention because the bleeding risks are very high and there is no documented benefit.” In these patients, an individual risk assessment is necessary to determine whether anticoagulation is appropriate or not in each case, the speaker said.
* CrCl = creatinine clearance
Congress: SGAIM Spring Congress 2021
Literature:
- Fehr T: DOAKs & renal insufficiency, Prof. Dr. med. Thomas Fehr, SGAIM Spring Congress, 19-21.05.2021
- Swissmedic: Medicinal Product Information, www.swissmedicinfo.ch (last accessed 11.06.21).
- The Task Force for the diagnosis and management of atrial fibrillation of the ESC. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal 2020; 42: 373-498.
- Hohnloser SH, et al: Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. European heart journal 2012; 33: 2821-2830.
- Rosemann A: New/Direct Anticoagulants (DOAK), last modified 11/2020, www.hausarztmedizin.uzh.ch (last accessed 11/06/2021).
- Stamellou E, Floege J: Novel oral anticoagulants in patients with chronic kidney disease and atrial fibrillation. Nephrol Dial Transplant 2018; 33(10): 1683-1689.
HAUSARZT PRAXIS 2021; 16(7): 23 (published 6/28/21, ahead of print).