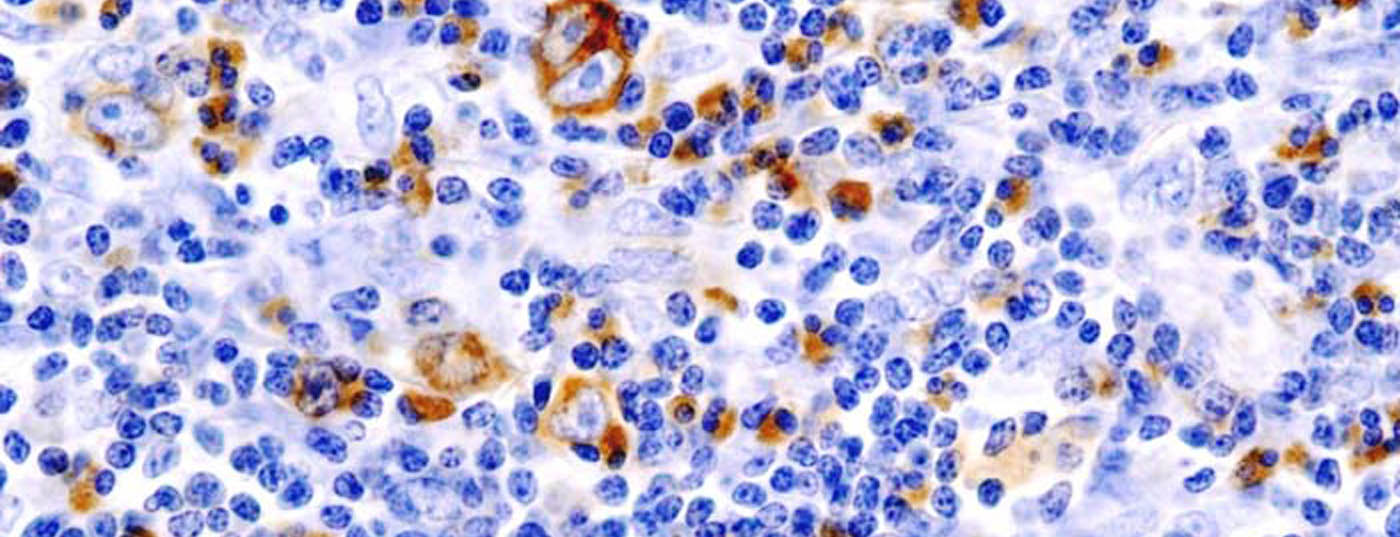
New results from a phase II study presented at the ASH Congress in New Orleans offer hope for patients with refractory or relapsed Hodgkin’s lymphoma: Approximately 10% of participants in this study were able to complete therapy and are living in long-term remission. This raises the question of whether brentuximab vedotin could provide a cure in a proportion of patients, making research into the compound as part of initial therapy all the more important.
“Overall, in this phase II study, half of the patients with refractory or relapsed Hodgkin lymphoma treated with brentuximab vedotin were alive at three years,” said Prof. Ajay K. Gopal, MD, Washington [1]. “More promising, however, is the finding that about 10% of patients were able to complete therapy and experience long-term remission.” This suggests a curative treatment option.
Hodgkin’s lymphoma can be characterized by CD30-positive Reed-Sternberg cells. The standard of care for refractory and relapsed Hodgkin lymphoma is chemotherapy followed by autologous stem cell transplantation. The nearly 50% of patients who experience relapse after autologous stem cell transplantation represent a population in urgent need of new therapeutic options. “A retrospective analysis of 756 patients shows that the median overall survival of these affected individuals is 2.4 years. Consequently, our Phase II study, which is currently still ongoing, aims to evaluate the efficacy and safety of brentuximab vedotin (Adcetris®) in 102 patients with refractory or relapsed Hodgkin’s lymphoma after autologous stem cell transplantation,” Prof. Gopal explained.
Long-term emission possible?
Brentuximab vedotin is a monoclonal antibody (anti-CD30 plus cytostatic agent monomethylauristatin E). In this study, it was used for a median of nine cycles (1.8 mg/kgKG every three weeks), in a patient population with a median age of 31 years, predominantly female (54%), and with an ECOG performance status of 1 (ECOG: scale indicating disease limitation).
Primary and Secondary Endpoints: The primary endpoint was the overall objective response rate. Secondary endpoints included complete remission, duration of response, progression-free and overall survival, and safety aspects.
Results: The objective overall response rate at the time of analysis (after just over three years of follow-up) was 75%. One-third of patients achieved complete remission. The median overall survival was 40.5 months and 5.6 months for progression-free survival. “Exactly half of the patients (51 of 102) were alive after a median observation time of 32.7 months since the first dose of brentuximab vedotin,” Prof. Gopal added.
According to the central independent review, 14 patients are currently in remission. They will continue to be monitored. Five of them received a consolidating allogeneic stem cell transplant. The remaining nine did not undergo additional treatment after brentuximab vedotin administration.
On median, these 14 patients were younger than those who did not respond to therapy. They also took more cycles (13.5 vs. 7).
Side effects: Side effects were predominantly fatigue (46%), peripheral sensory neuropathy (47%), and nausea (42%), at all grades. 14% of patients developed grade 3, 6% grade 4 neutropenia.
Promising results
“These 14 patients, with no evidence of lymphoma progression to date, offer evidence, albeit early, of a potential cure. A randomized phase III trial is under development evaluating brentuximab vedotin in combination with doxorubicin, vinblastine and dacarbazine versus doxorubicin, bleomycin, vinblastine and dacarbazine as first-line therapy,” Prof. Gopal concluded.
Source: 55th ASH Annual Meeting, December 7-10, 2013, New Orleans.
Literature:
- Gopal AK, et al: Three-Year Follow-Up Data And Characterization Of Long-Term Remissions From An Ongoing Phase 2 Study Of Brentuximab Vedotin In Patients With Relapsed Or Refractory Hodgkin Lymphoma. ASH Abstract # 4382.
CONGRESS SPECIAL 2014; 5(2): 2-3