Psoriasis in highly visible locations is particularly stigmatizing for patients. There is a high demand for effective and safe treatment options, especially for severe forms of the disease. Of those biologics that meet these criteria, 3-year data are available for one representative of the IL23p19 inhibitors, among others.
Back to “Atopic dermatitis and psoriasis news”.
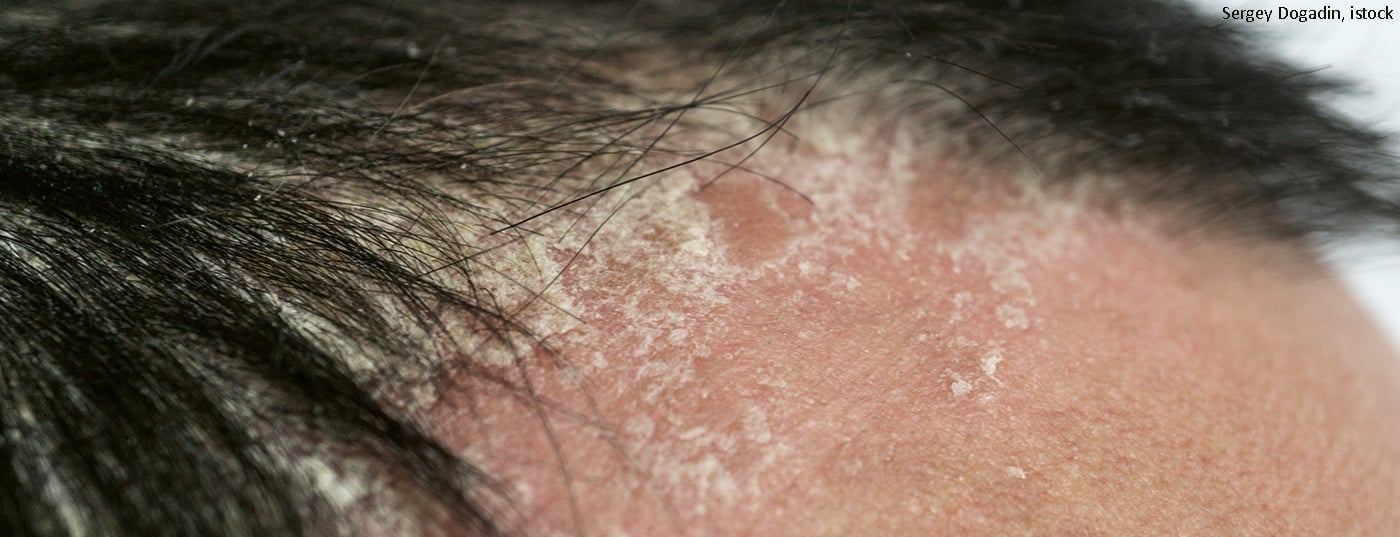
In a large proportion of patients with plaque psoriasis, the scalp, nails and palmoplantar areas are also affected. Infestation of the toenails and fingernails (psoriasis unguium) occurs in about half of patients and is often associated with severe disease progression. In terms of differential diagnosis, differentiation from mycotic diseases is particularly relevant. Psoriasis capitis is also common and is the first manifestation of the disease in about a quarter of patients. Plaques occur on the entire hairy head area and usually extend to the hairline, sometimes even to the forehead area, and the ears can also be affected.
Frequent and stigmatizing psoriasis manifestations.
Psoriatic symptoms in highly visible parts of the body may be associated with a particularly high psychological burden [1]. Especially in severe courses, the symptoms often prove to be very persistent. If topical treatment and conventional systemic therapeutics are not effective, the use of biologics should be considered. Compared to conventional systemic agents such as methotrexate, acitretin, ciclosporin or fumaric acid esters, the monoclonal antibodies are often not only more effective but also better tolerated. To evaluate the efficacy of the IL23p19 inhibitor risankizumab for the long-term treatment of nail psoriasis, psoriasis capitis, and palmoplantar areas, data from the randomized-controlled LIMMitless trial [2] were analyzed. For this purpose, subgroups of patients in the open-label extension phase of this study were identified who had corresponding symptoms at difficult-to-treat sites. This was operationalized using the Nail Psoriasis Severity Index (NAPSI)>0, Psoriasis Scalp Severity Index (PSSI)<0, Palmoplantar Psoriasis Severity Index (PPASI)>0. 525 of the total 598 study participants met these criteria and were included in further data analyses. Scalp psoriasis was present in over 90% of this subsample, nail involvement was present in over half, and palmoplantar psoriasis symptoms were present in about one-third. Percentage improvement in NAPSI, PSSI, and PPASI scores compared with baseline, as well as achievement of complete lesion freedom, were evaluated.
Patients benefit from lasting and significant relief of symptoms
Risankizumab therapy at a 12-week dosing interval resulted in excellent treatment outcomes. The mean improvement in NAPSI was over 70% at week 52 compared with baseline and remained continuously at this level until the end of the study period (172 weeks). With regard to PSSI, an improvement of over 90% was already evident at week 16, and these values also remained stable over the entire period. Also showing rapid improvement in a large proportion of study participants were PPASI scores: at week 16, this affected over 75% of patients, with this proportion even rising to over 90% at week 52 and remaining in this range until the end of the measurement period. Complete freedom from scalp lesions (PSSI) was achieved by more than 75% of patients as early as week 16, and by week 76 this value even increased to more than 90%. Also as of week 16, over 70% were free of palmplant symptoms (PPASI) and with regard to nail infestation (NAPSI), over half of the patients achieved complete clearance as of week 52. All these values remained stable over the entire study period of more than 3 years. Also recorded were changes in quality of life (Achievement of Dermatology Life Quality Index, DLQI). In this respect, too, the results are extremely favorable. As early as week 16, about two-thirds of the patients achieved a DLQI0/1, and by week 52, this score was over three-quarters. At week 172, more than 80% of study participants scored DLQI0/1.
Risankizumab treatment demonstrated a favorable safety profile throughout the more than three-year period with no emergence of new safety signals.
Summary
- Long-term treatment with the IL23p19 inhibitor risankizumab was shown to be highly effective in an interim analysis of the LIMMitless trial.
- The extent of psoriasis infestation of nails (NAPSI), scalp (PSSI) and palmoplantar areas (PPASI) reduced rapidly and permanently. Symptom reduction persisted throughout the study period of over 3 years.
- As early as week 16, about one-third of patients achieved DLQI0/1. Considering the particularly high psychological stress caused by these psoriasis manifestations, this is very significant for those affected.
Source: EADV Annual Meeting 2020
Literature:
- Lanna C, et al: Psoriasis in difficult to treat areas: treatment role in improving health-related quality of life and perception of the disease stigma. Journal of Dermatological Treatment 2020, https://doi.org/10.1080/09546634.2020.1770175
- Elewski BE, et al: Efficacy and Safety of Long-Term Risankizumab Treatment for Nail, Scalp, and Palmoplantar Psoriasis: An Interim Analysis from the Open-Label Extension LIMMitless Trial. Presentation ID FC03.09. Free Communications (On-demand sessions), EADV Virtual Congress 2020.
DERMATOLOGIE PRAXIS 2021; 31(1): 38 (published 2/23/21, ahead of print).