The range of interstitial lung disease is wide. Therapeutic progress has been made in many areas over the past decade. In idiopathic pulmonary fibrosis (IPF), there was virtually no way to offer meaningful therapy to affected patients until the early 2010s. However, drugs are now available that significantly delay disease progression.
In May, the German-language guideline for the diagnosis of ILD was renewed. “Interstitial lung disease is a term we still use, although diffuse parenchymal disease of the lung would fit the picture much better,” said Prof. Michael Pfeifer, M.D., head of the Department of Pneumology at Regensburg University Hospital (D), by way of introduction. He presented the case history of one of his patients, demonstrating how the diagnostic procedure is classically performed according to the guidelines. He and a colleague paid special attention to the differential diagnosis from a radiological point of view.
Patient was exposed to isocyanates
A 62-year-old patient presented to Prof. Pfeifer in September 2016 with a history of interstitial lung disease. The main symptom was exertional dyspnea, but also an increased irritable cough. Otherwise, the man offered no significant abnormalities, neither fever nor weight loss, nor were joint complaints, skin changes or sicca symptoms noted.
Exposures to, for example, pets or mold in the home-family environment were not present; however, the patient worked as a plastic molder and thus was exposed to isocyanates and fumes. In the past, a rehabilitation clinic had already sought recognition as an occupational disease, but this was rejected. The man had allergies to grass pollen, but otherwise had no particular risk profile. He did not drink alcohol and had been a non-smoker since 1989, prior to that he had 10 Pack Years.

Pre-existing conditions were known to be arterial hypertension and bronchial asthma for 15 years, but the latter was asymptomatic and not prominent at the time of presentation. He had obstructive sleep apnea syndrome with nCPAP therapy and nasal surgery the previous year. The patient was taking verapamil 120 mg, valsartan 160 mg, Symbicort 320/9 μg, and salbutamol.
On examination, bilateral basal inspiratory crackles were noted; otherwise, no special abnormalities were found apart from obesity and elevated blood pressure. A restriction in the flow-volume curve was visible on pulmonary function measurement. TLC was 73.5%, VC was 70.7%, and FVC was 64.1%. The diffusion capacity showed a minor limitation.
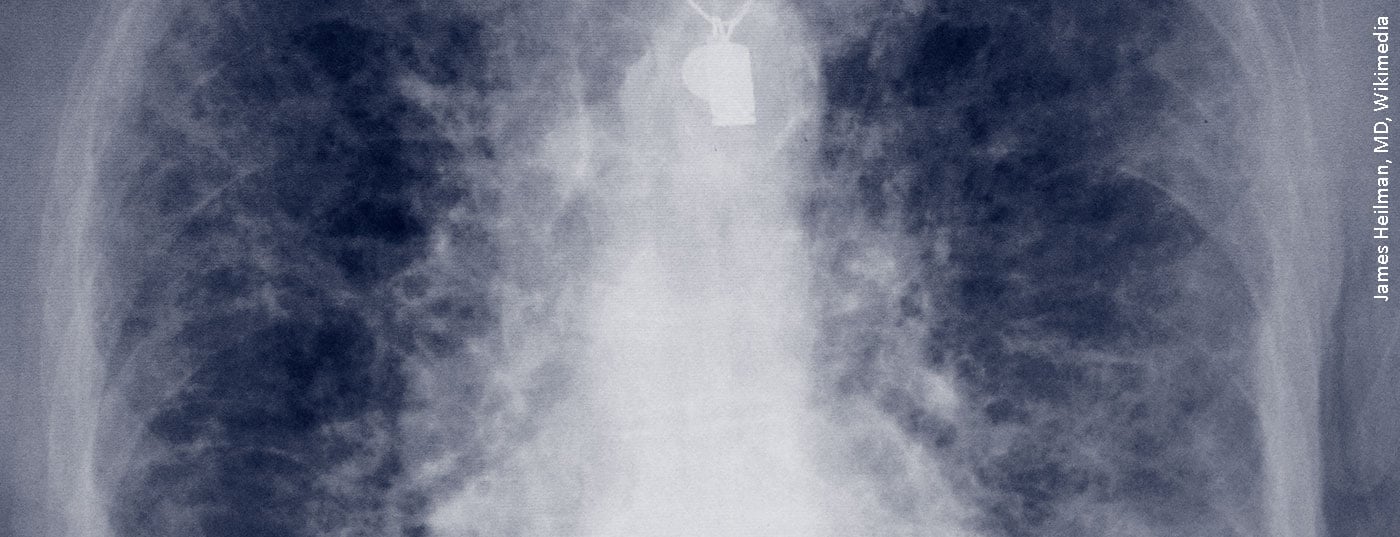
Radiological clarification in HRCT
High-resolution computed tomography (HRCT) was performed in the Department of Radiology at Donaustauf Hospital. Prof. Okka Hamer, M.D., chief of radiology, pointed out that this thin-slice CT should be the standard of care when evaluating ILD. HRCT showed reticulations bilaterally, involving the periphery of the lung. “This image would be classified today as probable, meaning probable UIP,” Prof. Hamer said, due to the reticulations, traction broncheolectasis, lack of safe honeycombs and missing signs that argued against a UIP pattern. Four years ago, colleagues on the Board for Interstitial Lung Disease named it possible, meaning possible UIP (Usual Interstitial Pneumonia), according to the classification at the time. However, non-specific interstitial pneumonia (NSIP) was also discussed as a differential diagnosis. Transbronchial cryobiopsy and rheumatologic presentation were recommended for further evaluation.
Rheumatology laboratory showed ANA titer at 1:320, rheumatoid factor was 26.6 IU/ml (<15.0). Despite these abnormalities, however, no clear evidence of inflammatory rheumatic systemic disease was found at rheumatology presentation.
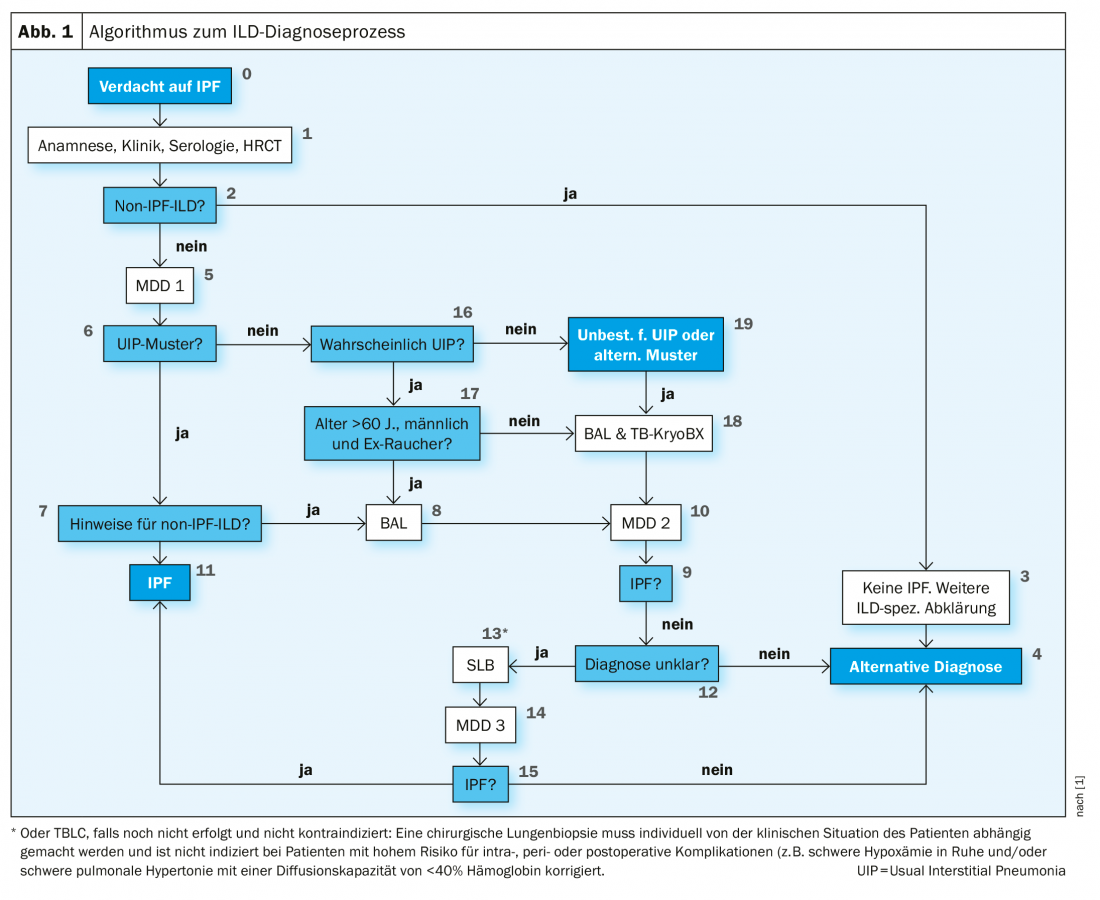
The procedure of the physicians in 2016 also corresponds to the requirements of the current S2k guidelines of the German-speaking countries (Fig. 1). These state that a bronchoalveolar lavage (BAL) and a cryobiopsy should be performed in patients with suspected IPF and an undetermined or alternative HRCT pattern for UIP. Prof. Pfeifer noted that, in contrast, international guidelines are still very critical of cryobiopsy. It is listed but only recommended in very experienced centers. In many countries, VATS (video-assisted thoracoscopic surgery) remains the method of first choice.
Therapy brought stabilization
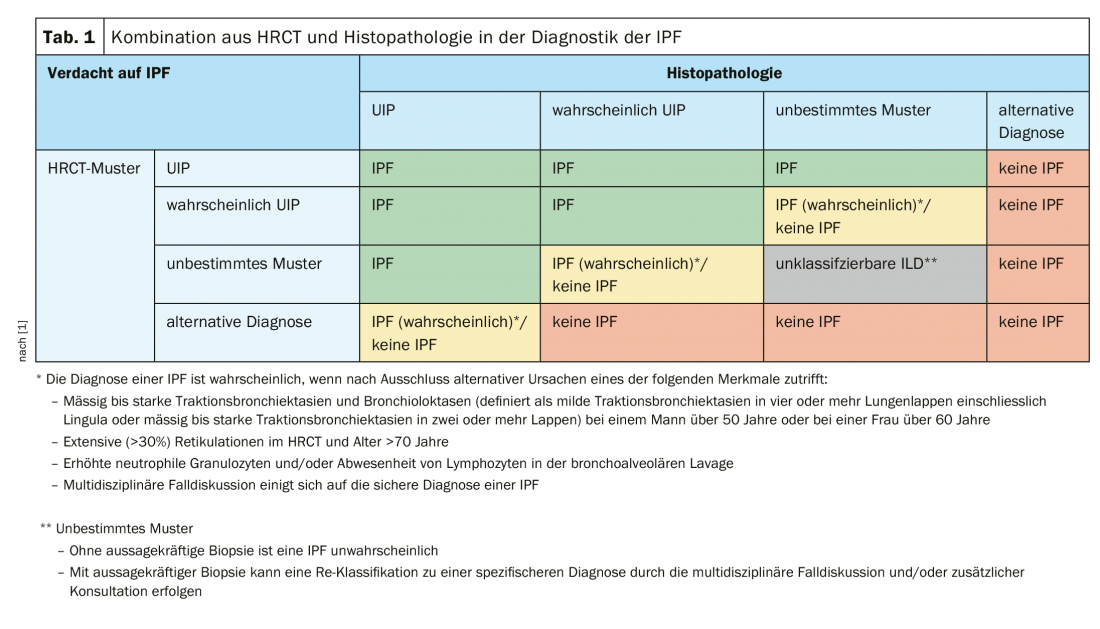
However, histologic confirmation was also not possible by cryobiopsy in the patient. Likewise, a BAL that was performed did not lead to the goal. In the following ILD board it was then decided to conduct a VATS. In this case, the upper lobe showed panacinar emphysema on the one hand and the picture of chronic hypersensitivity pneumonia on the other hand, possibly in the context of occupational exposure to isocyanate. In the lower lobe, the full pattern of UIP with honeycombing and fibroblastic nodules was found. This ultimately led to a third, final ILD board in which the presence of IPF was assumed. The physicians recommended initiation of antifibrotic therapy with pirfenidone or nintedanib. This procedure also reflects the recommendations of the S2k guideline, which stipulate a combination of HRCT and histopathology in the diagnosis of IPF (Table 1).
Under therapy, significant stabilization without progression was observed between early 2017 and early 2019. However, in March 2019, the patient was diagnosed with myelodysplastic syndrome with blast excess (MDS-EB 1) during a febrile episode. Nevertheless, therapy with nintedanib was continued after consultation with the hematooncologist, but the patient ultimately died of the disease. However, the treating physicians could not find any evidence of a causal relationship between the antifibrotic therapy and the occurrence of the malignant blood disease.

In summary, this case report is an almost classic case that can be used as an example to illustrate how such a case can be processed in a structured manner in daily work according to the guidelines in order to classify it and initiate therapy on the basis of this classification.
Source: Online seminar “Differential diagnosis and therapy in ILD”, Boehringer Ingelheim Partner’s Satellite, streamed-up.com
Literature:
- Behr J, et al: Pneumology 2020; 74(5): 263-293; doi: 10.1055/a-1179-2905.
InFo PNEUMOLOGY & ALLERGOLOGY 2020; 2(3): 28-30 (published 9/22/20, ahead of print).