Pyoderma gangraenosum is an immunologically induced wound, but the exact pathogenesis is not yet clear. The Paracelsus score is an important validated tool for the diagnosis of these extremely painful ulcerations, which occur preferentially on the extensor sides of the lower legs. The therapy is based on several pillars, with systemic treatment being of central importance.
Clinically, pyoderma gangraenosum (PG) presents as painful ulcerations with a rim that is often undermined by livid erythema. “It is very important that we have many differential diagnoses that look similar,” said Prof. Joachim Dissemond, MD, Clinic and Polyclinic for Dermatology, Venereology and Allergology, University Hospital Essen [1]. PG is classified as a rare, primary sterile, neutrophilic dermatosis. The quality of life of those affected can be significantly impaired and it should be remembered that PG is potentially life-threatening [2].
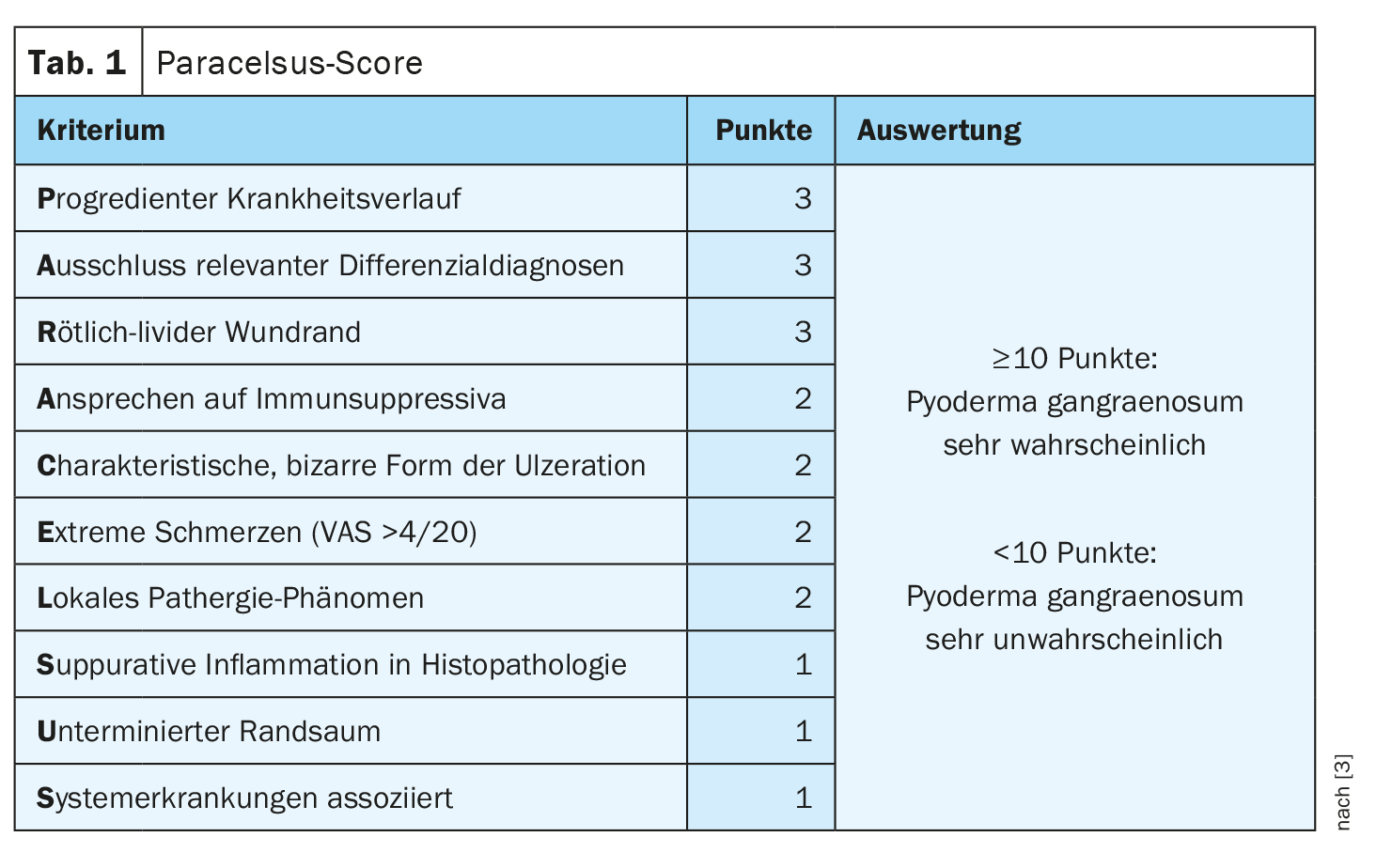
In case of suspected diagnosis, the Paracelsus score is helpful
Inflammation is a central component of PG, and the inflammatory aspect is often confused with infection, which can lead to the erroneous use of antibiotics. “We try to rule out differential diagnoses,” adds Prof. Dissemond. A PG can basically develop anywhere, but there is a clear predilection site: in 70% of cases the extensor sides of the lower legs are affected. In women, the second most common location is the chest area. “Often, pyoderma occurs where there has been trauma,” although it can be minor injuries, the speaker explained. Important differential diagnoses with similar appearance to PG but different therapy include vasculitis, livedovasculopathy, calciphylaxis, hypertonic ulcer Martorell, and artificial wounds.
The Paracelsus score is currently considered the best validated diagnostic score [3]. Three points are awarded for a main criterion, two points for a secondary criterion and one point each for an additional criterion. An additive score of ≥10 makes the presence of a PG very likely (Table 1). A progressive disease course and exclusion of relevant diagnoses are the first two main criteria. The reddish-livid rim is a very important but non-specific feature for inflammation. “Since it is an autoinflammatory condition, it should respond to immunosuppression,” explained Prof. Dissemond. Morphologically, a characteristically bizarre shape of the ulceration is striking. Also a Paracelsus criterion is the extreme painfulness. “Pyoderma gangraenosum hurts,” the speaker emphasizes. Regarding the local pathergy phenomenon – a disease-specific skin reaction to a non-specific stimulus – it should be noted that it can also be negative. To perform the pathergy test, 1 ml of 0.9% NaCl is injected. If a pustule, redness, or neutrophil-rich inflammation develops within 48 hours, this can be considered a positive pathergy phenomenon [2]. Histopathological examination should also not be omitted. Biopsies are usually taken in the marginal area of the ulceration, and a small narrow spindle biopsy is best suited, the speaker said. Suppurative inflammation on histopathology is suggestive of PG. However, it should be noted that the findings could also remain unclear. An undermined livid rim is an important differential diagnostic feature to livedovasculopathy and another Paracelsus criterion [4]. Furthermore, association with other systemic diseases is known to be common in PG.
Pyoderma gangraenosum is often associated with comorbidities
9-36% of patients have inflammatory bowel disease (ulcerative colitis, Crohn’s disease) and 8-33% have rheumatoid arthritis. These comorbidities are no coincidence, Prof. Dissemond knows. “We are talking about a systemic inflammatory disease that has many similarities to other autoiinfammatory symptoms.” What is often forgotten, he said, is that it is a potentially paraneoplastic disease. 4-21% of patients have concomitant hematologic neoplasia (myelodysplastic syndrome, renal cell carcinoma). Therefore, it would be worthwhile to do a differential blood count. In addition to these associations, which have been known for many years, there is apparently also an increased incidence of various diseases from the metabolic syndrome group (e.g. arterial hypertension, diabetes mellitus, lipid metabolic disorder) [2]. In addition, there are manifestations of PG in the setting of autoinflammatory diseases (e.g., PAPA syndrome, PASH syndrome, PA-PASH syndrome).

System therapy is the be-all and end-all
Due to the rarity of the disease and lack of evidence in the form of randomized clinical trials (RCTs), there is no uniform standard of care for pyoderma gangraenosum, but the guideline makes recommendations based on case reports and clinical expertise (Fig. 1) [2]. Immunosuppressive systemic therapy is indicated in most patients with PG, especially in cases of severe disease activity and multiple lesions or after failure of topical therapy [2]. Among systemic therapeutics, glucocorticoids (prednisone, 0.5-1 mg/kg bw) are most commonly used, followed by ciclosporin (2-5 mg/kg bw), as monotherapy or in combination [5]. Downstream systemic therapies that are also commonly used include TNFα inhibitors, azathioprine (100-150 mg/d), or mycophenolate mofetil [5]. Supplementary to this, resp. in initial and mild disease progression, topical and local intralesional therapy options are used. In the acute phase, one can use a topical corticosteroid of strength class III or IV or a topical calcineurin inhibitor. However, system therapy is clearly the most important treatment pillar. However, the guideline advises against longer-term monotherapy with systemic glucocorticoids because of the adverse side effects [2]. Therefore, or in case of insufficient efficacy of monotherapy with glucocorticoids, early combination with steroid-sparing immunosuppressants such as ciclosporin or azathioprine or TNF-α inhibitors or else monotherapy with TNF-α inhibitors is recommended. The monoclonal antibodies infliximab, adalimumab, golimumab and certolizumab pegol or etanercept are the therapy of choice when PG occurs as a concomitant disease of rheumatoid arthritis or inflammatory bowel disease. Otherwise, these TNF-α antagonists are off-label use. Intravenous immunoglobulins (0.5-2 g/kg bw) are also recommended as an immunomodulatory therapy option in the guideline, although this is a relatively costly therapy [1,2]. In addition, the guideline identifies several other systemic therapeutics for consideration (Fig. 1) .
Congress: Wound Congress Nuremberg
Literature:
- “Update Pyoderma gangraenosum”, Prof. Dr. Joachim Dissemond, Wound Congress Nuremberg, 01.12.2022.
- S1-Leitlinie «Pyoderma gangrenosum», AWMF-Register-Nr.: 013–091, 2020, https://register.awmf.org/assets/guidelines/013-091l_S1_Pyoderma-gangre
nosum_2020-10_1.pdf, (last accessed 13.12.2022). - Jockenhöfer F, et al.: The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol 2019; 180: 615–620.
- Schiffmann ML, et al. S1-Leitlinie Diagnostik und Therapie der Livedovaskulopathie. J Dtsch Dermatol Ges 2021; 19(11): 1667–1678.
- Al Ghazal P, Dissemond J: Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges. 2015; 13: 317–324.
HAUSARZT PRAXIS 2023; 18(1): 30–31 (published 1/26/2013, ahead of print).